- paracetamol,
- hydrogen bonding interactions,
- natural bond orbital (NBO),
- quantum theory of atoms in molecules (QTAIM)
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
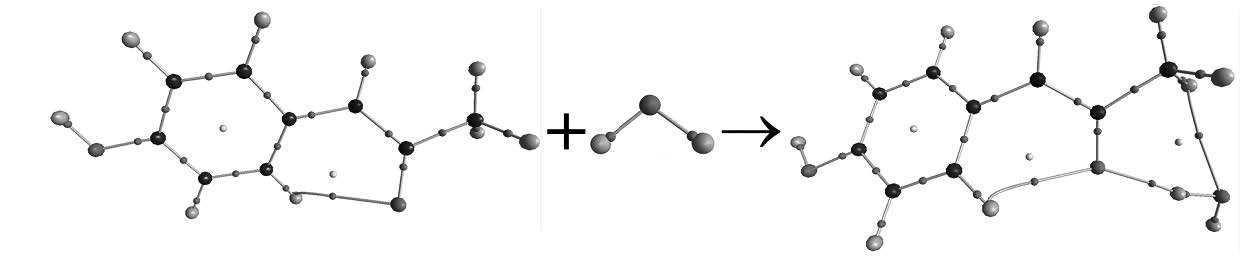
The paracetamol–water (PA–H2O) complexes formed by hydrogen bonding interactions were investigated at the MP2/6–311++G(d,p) level. Six PA–H2O complexes possessing various types of hydrogen bonds (H–bonds) were characterized by geometries, energies, vibrational frequencies. Natural bond orbital (NBO), quantum theory of atoms in molecules (QTAIM) and the localized molecular orbital energy decomposition analysis (LMO–EDA) were performed to explore the nature of the hydrogen–bonding interactions in these complexes. The intramolecular H–bond formed between the methylene and carbonyl oxygen atom of paracetamol is retained in most of complexes. The H–bonds in PW1 and PW6 are stronger than other H–bonds, moreover, the researches show that both the hydrogen bonding interaction and structural deformation play important roles for the relative stabilities of PA–H2O complexes.
References
- M. Sciskalska, M. Sliwinska-Mosson, M. Podawacz, W. Sajewicz and H. Milnerowicz, Drug. Chem. Toxicol. 38, 121 (2015).
- W.S. Waring, H. Jamie and G.E. Leggett, Hum. Exp. Toxicol. 29, 63 (2010).
- M. Naggayi, N. Mukiibi and E. Iliya, Afr. Health. Sci. 15, 598 (2015).
- S.B.K. Mahadevan, P.J. McKiernan, P. Davies and D.A. Kelly, Arch. Dis. Child. 91, 598 (2006).
- M. Cekmen, Y.O. Ilbey, E. Ozbek, A. Simsek, A. Somay and C. Ersoz, Food Chem. Toxicol. 47, 1480 (2009).
- P. Abraham, Nephrology. 10, 623 (2005).
- N.V. Nayyer, J. Byers and C. Marney, Brit. Dent. J. 216, 229 (2014).
- P. Marzuillo, S. Guarino and E. Barbi, Eur. J. Pediatr. 173, 415 (2014).
- A.-R. Marzilawati, Y.-Y. Ngau and S. Mahadeva, BMC Pharmacol. Toxico. 13, 8 (2012).
- H. Jaeschke, Digest. Dis. 33, 464 (2015).
- E.W. Holt, S. DeMartini and T.J. Davern, J. Clin. Gastroenterol. 49, 790 (2015).
- G.G. Graham, M.J. Davies, R.O. Day, A. Mohamudally and K.F. Scott, Inflammopharmacology. 21, 201 (2013).
- M.L. Ramos, J.F. Tyson and D.J. Curran, Anal. Proc. incl. Anal. Comm. 32, 175 (1995).
- P.A. Mosier-Boss, S.H. Lieberman and R. Newbery, Appl. Spectrosc. 49, 630 (1995).
- B.B. Ivanova, J. Mol. Struct. 738, 233 (2005).
- I.G. Binev, P. Vassileva-Boyadjieva and Y.I. Binev, J. Mol. Struct. 447, 235 (1998).
- E. Dreassi, G. Ceramelli, P. Corti, M. Massacesi and P.L. Perruccio, Analyst. 120, 2361 (1995).
- J.M. Beames and A.J. Hudson, Phys. Chem. Chem. Phys. 12, 4157 (2010).
- Y. Danten, T. Tassaing and M. Besnard, J. Phys. Chem. A. 110, 8986 (2006).
- S.J. Lee, A. Min, Y. Kim, A. Ahn, J. Chang, S.H. Lee, M.Y. Choi and S.K. Kim, Phys. Chem. Chem. Phys. 13, 16537 (2011).
- M. Yoosefian and N. Etminan, RSC Adv. 5, 31172 (2015).
- S. Rai, H. Singh and U.D. Priyakumar, RSC Adv. 5, 49408 (2015).
- K.H. Moller, A.S. Hansen and H.G. Kjaergaard, J. Phys. Chem. A. 119, 10988 (2015).
- M. Izadyar, M. Khavani and M.R. Housaindokht, Phys. Chem. Chem. Phys. 17, 11382 (2015).
- Z.G. Huang, Y.M. Dai and L. Yu, Struct. Chem. 21, 863 (2010).
- L.F. Guo, Z.G. Huang, T.T. Shen, L.L. Ma and X.Q. Niu, Chin. J. Chem. 31, 1079 (2013).
- P.L.A. Popelier: Atoms in Molecules: An Introduction (Prentice Hall, City, 2000).
- C.F. Matta and R.J. Boyd: The Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design (WILEY-VCH Verlag GmbH & Co. KGaA, City, 2007).
- A.E. Reed, L.A. Curtiss and F. Weinhold, Chem. Rev. 88, 899 (1988).
- P.F. Su and H. Li, J. Chem. Phys. 131, 014102 (2009).
- J. Xi and X. Xu, Phys. Chem. Chem. Phys. 18, 6913 (2016).
- S.K. Singh, S. Kumar and A. Das, Phys. Chem. Chem. Phys. 16, 8819 (2014).
- J.J. Panek, A. Filarowski and A. Jezierska-Mazzarello, J. Chem. Phys. 139, 154312 (10 pp.) (2013).
- A. Lakshmipriya, S.R. Chaudhari, A. Shahi, E. Arunan and N. Suryaprakash, Phys. Chem. Chem. Phys. 17, 7528 (2015).
- M. Jablonski and M. Palusiak, J. Phys. Chem. A. 116, 2322 (2012).
- L.L. Ma, Z.G. Huang, X.Q. Niu, T.T. Shen and L.F. Guo, Comput. & Theor. Chem. 1017, 14 (2013).
- E. Espinosa, I. Alkorta, J. Elguero and E. Molins, J. Chem. Phys. 117, 5529 (2002).
- U. Koch and P.L.A. Popelier, J. Phys. Chem. 99, 9747 (1995).
- W.D. Arnold and E. Oldfield, J. Am. Chem. Soc. 122, 12835 (2000).
- S. Jenkins and I. Morrison, Chem. Phys. Lett. 317, 97 (2000).
- S.J. Grabowski, W.A. Sokalski and J. Leszczynski, J. Phys. Chem. A. 110, 4772 (2006).
- L.F. Pacios, J. Phys. Chem. A. 108, 1177 (2004).
- A.E. Reed, F. Weinhold, L.A. Curtiss and D.J. Pochatko, J. Chem. Phys. 84, 5687 (1986).
- M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery Jr., J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, Ö. Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski and D.J. Fox: Gaussian09, (Gaussian, Inc., City, 2009).
- F. Biegler-König and J. Schönbohm: AIM2000, (University of Applied Sciences, City, 2000).
- M.W. Schmidt, K.K. Baldridge, J.A. Boatz, S.T. Elbert, M.S. Gordon, J.H. Jensen, S. Koseki, N. Matsunaga, K.A. Nguyen, S. Su, T.L. Windus, M. Dupuis and J.A. Montgomery, J. Comput. Chem. 14, 1347 (1993).
- H.K. Wang, Z.G. Huang, T.T. Shen and L.F. Guo, Struct. Chem. 23, 1163 (2012).
- H.K. Wang, Z.G. Huang, T.T. Shen and L.F. Guo, J. Mol. Model. 18, 3113 (2012).
- L. Yu, Y.H. Wang, Z.G. Huang, H.K. Wang and Y.M. Dai, Int. J. Quantum Chem. 112, 1514 (2012).
- X.Q. Niu, Z.G. Huang, L.L. Ma, T.T. Shen and L.F. Guo, J. Chem. Sci. 125, 949 (2013).
- Z.G. Huang, L. Yu, Y.M. Dai and H.K. Wang, Struct. Chem. 22, 57 (2011).
- Z.G. Huang, L. Yu and Y.M. Dai, Int. J. Quantum Chem. 111, 3915 (2011).
- Z.G. Huang, Y.M. Dai, L. Yu and H.K. Wang, J. Mol. Model. 17, 2609 (2011).
- Z.G. Huang, L. Yu, Y.M. Dai and H.K. Wang, J. Mol. Struct. (Theochem). 960, 98 (2010).
- Z.G. Huang, L. Yu and Y.M. Dai, Struct. Chem. 21, 855 (2010).


