Vol 61 No 1 (2016): Journal of the Chilean Chemical Society
Original Research Papers
Published
December 10, 2015
Keywords
- Phenacyl bromide,
- Quinoxaline,
- Ionic liquids,
- Catalyst,
- Green chemistry
How to Cite
TEJESWARARAO, D. (2015). RECYCLABLE ACIDIC BRØNSTED IONIC LIQUID CATALYZED SYNTHESIS OF QUINOXALINE. Journal of the Chilean Chemical Society, 61(1). Retrieved from https://jcchems.com/index.php/JCCHEMS/article/view/55
Copyright (c) 2016 D. TEJESWARARAO

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Abstract
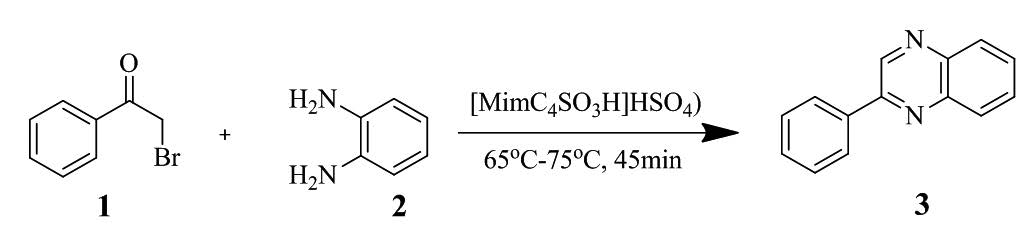
The acidic ionic liquid, 1-(4-sulfonic acid) butyl-3-methylimidazolium hydrogen sulfate [MimC4SO4H]HSO4 catalyzed two-component condensation reaction of phenacyl bromide and o-phenylenediamine to afford corresponding quinoxaline derivatives. The inexpensive and non-toxic ionic liquids can be reused several times without any perceptible loss of their activities.
References
- -Ali M. M., Ismail M. M. F., EI-Gabby M. S. A., Zahran M. A., Ammar T. A., Molecules, 5, 864, (2000).
- -Sarges R., Howard H. R., Browne R. C., Label L. A., Seymour P. A., J. Med. Chem., 33, 2240, (1990).
- -Sakata G., Makino K., Kurasawa Y., Heterocycles, 27, 2481, (1998).
- -Arthur G., Elor K. B., Robert G. S., Guo Z. Z., Richard J. P., Stanley D., John R. K., Sean T., J. Med. Chem., 48, 744, (2005).
- -Singh, S. K.; Saibaba, V.; Ravikumar, V.; Rudrawar, S. V.; Daga, P.; Rao, C. S.; Akhila, V.; Hegde, P.; Rao, Y. K. Bioorg. Med. Chem. 12, 1881, (2004).
- -Katoh, A.; Yoshida, T.; Ohkanda, J. Heterocycles, 52, 911, (2000).
- -Thomas, K. R. J.; Velusamy, M.; Lin, J. T.; Chuen, C.-H.; Tao, Y.-T. Chem. Mater., 17, 1860, (2005).
- -Dailey, S.; Feast, W. J.; Peace, R. J.; Sage, I. C.; Till, S.; Wood, E. L. J. Mater. Chem. 11, 2238, (2001).
- -Sascha, O.; Ru¨ diger, F. Synlett 1509, (2004).
- -Sessler, J. L.; Maeda, H.; Mizuno, T.; Lynch, V. M.; Furuta, H. J. Am. Chem. Soc. 124, 13474, (2002).
- -Crossley, M. J.; Johnston, L. A. Chem. Commun. 1122, (2002).
- -Dell, A.; William, D. H.; Morris, H. R.; Smith, G. A.; Feeney, J.; Roberts, G. C. K. J. Am. Chem. Soc. 97, 2497, (1975).
- -Bailly, C.; Echepare, S.; Gago, F.; Waring, M. J. Anti-Cancer Drug Des. 15, 291, (1999).
- -Raw, S. A.; Wilfred, C. D.; Taylor, R. J. K. Org. Biomol. Chem. 2, 788, (2004).
- -Aparicio, D.; Attanasi, O. A.; Filippone, P.; Ignacio, R.; Lillini, S.; Mantellini, F.; Palacios, F.; de los Santos, J. M. J. Org. Chem. 71, 5897, (2006).
- -Singh, S. K.; Gupta, P.; Duggineni, S.; Kundu, B. Synlett 2147, (2003).
- -Antoniotti, S.; Dun˜ach, E. Tetrahedron Lett. 43, 3971, (2002).
- -Mao, L. Sakurai, H. Hirao, T. Synthesis 2535, (2004).
- -Zhao, Z.; Wisnoski, D. D.; Wolkenberg, S. E.; Leister, W. H.; Wang, Y.; Lindsley, C.
- -W. Tetrahedron Lett. 45, 4873, (2004).
- -Jain N, Kumar A, Chauhan S & Chauhan, Tetrahedron 61, 1015, (2005).
- -Galinski M, Lewandowski A & Stepniak I, Electrochimica Acta 51, 5567, (2006).
- -R. M. Cowper and L. H. Davidson. Organic Syntheses, 5, 117, (2005).


