- Adsorption,
- 4–Nitrophenol,
- Supports,
- π-complexation
Copyright (c) 2016 C. Matus, E. Camú, M. Villarroel, J. Ojeda, P. Baeza

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Abstract
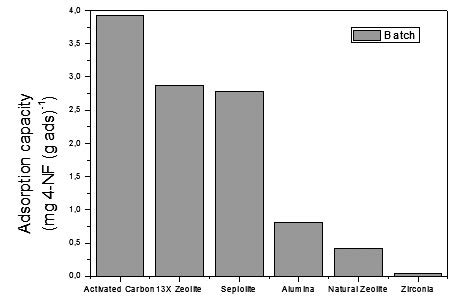
The removal of 4–Nitrophenol (4–NP) from aqueous media by adsorption is studied in a batch system using different porous materials: activated carbon, zirconia, alumina, sepiolite, natural zeolite and 13X zeolite. Depending on their adsorption capacities, the best adsorbent was chosen to be impregnated with different levels of nickel (Ni) in order to study the adsorption by π-complexation in batch and continuous systems. The samples of Ni(x)/support (x= 2, 4, 6%) were prepared by wet impregnation and were characterised using the same method as with all the materials, by N2 adsorption-desorption using the BET method, surface acidity and Z potential measurements by electrophoretic migration. The samples were measured in a UV-Vis electrophotometer at a wavelength of 318 nm, while the adsorption capacity of the material in the batch system was determined by calculating the difference in concentration once the adsorbent became saturated after an initial concentration of ~20 ppm, and in the continuous system this was done by integrating the area under the adsorption curve. The results suggest that adsorption capacity depends on the specific BET area, apparent acid strength and the IEP of each support, and that it varies with the addition of Ni.
References
- -Ramírez, J., Lacasaña, M., Archivo Prevención de Riesgos Laborales., 4(2), 67 – 75, (2001).
- -Ferrusquía-García, C., Revista Latinoamericana de Recursos Naturales., 4(2), 285 – 290, (2008).
- -Cavoski, I., J. Agric. Food. Chem., 56(17), 8066-8073, (2008).
- -Wang, L., Chi, X., Zhang, J., Sun, D., Zhou, N., Int. Biodeterior. Biodegrada., 17, 116-121, (2014).
- -Goi, A.,Trapido, M., Adv. Environ. Res., 8, 303-311, (2004).
- -Realini, P., J. Chromatogr. Sci., 19(3), 124-129, (1981).
- -Shea, P., Weber, J., Review., 87, 1-41, (1983).
- -Rubio, M., Lissi, E., Herrera, N., Pérez, V., Chemosphere., 86(10), 1035- 1039, (2012).
- -Parida, K., Prakasini, D. J., J. Photochem. Photobiol A., 163(3), 561-567, (2004).
- -Finkel, A., Herbicides: Dinitrophenols. In: Hamilton and Hardy’s Industrial Toxicology, 4ta Edición, Boston, John Wright PSG, (1983), 301.
- -Palmeira, C., Moreno, A., Toxicol Lett., 81,115-123, (1995).
- -Rafael Gómez Bachiller., Estudio de la degradación de Nitrofenoles mediante Fotocatálisis Heterogénea. Tesis (Licenciado en Química). Madrid, España, Universidad Carlos III, Departamento de Ciencias e Ingeniería de Materiales e Ingeniería Química, (2011), 65 h.
- -Luis Alfredo Aznate Teheran/ Degradación por fotocatálisis (Foto-Fenton) de efluentes líquidos contaminados con residuos de fenol.Tesis (Ingeniero Químico). Cartagena de Indias D. T y C., Colombia, Universidad de Cartagena, Facultad de Ingeniería, (2013), 87 h.
- -Baeza, P., Aguila, G., Gracia, F., Araya, P., Catal. Commun., 9, 751-755, (2008).
- -Baeza, P., Aguila, G., Vargas, G., Ojeda, J., Araya, P., Appl. Catal., B (111), 133-140, (2012).
- -Hernandez-Maldonado, A.J., Yang, R.T., AIChE Journal., 50(4), 791-801, (2004).
- -Cid, R., Pecchi, G., Appl. Catal., 14, 15-21, (1985).
- -Enríquez, J., Alamilla, R., García, U., Rodrigo, R., Quim. Nova., 36(7), 937-941, (2013).
- -Kwon, J., Wilson, L., J. Environ. Sci. Health., A (45), 1793-1803, (2010).


