- Phosphorus compound,
- DFT,
- ADF,
- Spectroscopic Studies
Copyright (c) 2016 Amir Lashgari, Shahriar Ghamami, Guillermo Salgado-Moran, Rodrigo Ramirez-Tagle, Lorena Gerli – Candia

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Abstract
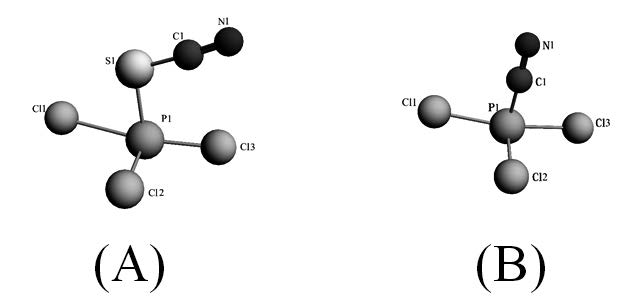
Two new phosphorus complexes, potassium trichlorothiocyanophosphate (III) (PTCTCP; K[PCl3(SCN)]) and potassium trichlorocyanophosphate (III) (PTCCP; K[PCl3(CN)]) were synthesized from the reaction of KSCN and KCN, respectively, with PCl3. The chemical formulas and compositions of these compounds were determined by elemental analysis and spectroscopic methods, such as phosphorus-31 nuclear magnetic resonance (NMR) spectroscopy (31P-NMR), Fourier transform infrared (FTIR) spectroscopy, ultraviolet-visible (UV-Vis) spectroscopy and mass spectrophotometry. All of the theoretical calculations and determinations of the properties of these compounds were performed as part of the Amsterdam Density Functional (ADF) program. Excitation energies were assessed using time-dependent perturbation density functional theory (TD-DFT). In addition, the molecular geometry was optimized and the frequencies and excitation energies were calculated using standard Slater-type orbital (STO) basis sets with triple-zeta quality double plus polarization functions (TZ2P) for all of the atoms. The assignment of the principal transitions and total densities of state (TDOS) for orbital analysis were performed using the GaussSum 2.2 program.
References
- -Edmond R.T.; Organophosphorus Complexes of Cobalt Carbonyl as Hydroformylation Catalysts, Ind. Eng. Chem. Prod. Res. Dev., 8 (3), 286–290, (1969).
- -Jeffrey M.J.; Allen, W.V.; Lewis, W.C.; John, H.N.; Palladium(II) thiocyanate organophosphorus complexes, Inorg. Chem., 19 (4), 1036– 1039, (1980).
- -Downing, J.H.; Smith, M.B. “Phosphorus Ligands”. Comprehensive Coordination Chemistry II, 253–296, (2003).
- -Balali-Mood, M.; Shariat, M. Treatment of organophosphate poisoning, Experience of nerve agents and acute pesticide poisoning on the effects of oximes. J. Physiol Paris, 92, 375 – 378, (1998).
- -Quin, L. D.; A Guide to Organophosphorus Chemistry; John Wiley & Sons, (2000).
- -Racke, K.D.; “Degradation of organophosphorus insecticides in environmental matrices”, pp. 47–73 in: Chambers, J.E., Levi, P.E. (eds.), Organophosphates: Chemistry, Fate, and Effects. Academic Press, San Diego, (1992).
- -Kabachnik, M. I.; Baranov, A. P.; Medved, T. Ya.; Theoretical conformational analysis of cyclopendant organophosphorus chelating agents, derivatives of cyclam, Theoretical and Experimental Chemistry, 26, 2, 169-174, (1990).
- -Jenkins, J.M.; Verkade, J.G.; Stereochemistry of organophosphorus complexes of transition metals, Inorg. Chem., 6 (12), 2250–2255, (1967).
- -Pan, B.; Evers-McGregor, D.A.; Bezpalko, M.W.; Foxman, B.M.; Thomas, C.M.; Multimetallic Complexes Featuring a Bridging N-heterocyclic Phosphido/Phosphenium Ligand: Synthesis, Structure, and Theoretical Investigation, Inorg. Chem., 52 (16), 9583–9589, (2013).
- -Purgel, M.; Baranyai, Z.; Blas, A.; Rodríguez-Blas, T.; Bányai, I.; Platas- Iglesias, C.; Tóth, I.; An NMR and DFT Investigation on the Conformational Properties of Lanthanide(III) 1,4,7,10-Tetraazacyclododecane-1,4,7,10- tetraacetate Analogues Containing Methylenephosphonate Pendant Arms, Inorg. Chem., 49 (9), 4370–4382, (2010).
- -Hai-Sheng, R.; Mei-Jun, M.; Jian-Yi, M.; Xiang-Yuan, Li.; Theoretical Calculation of Reorganization Energy for Electron Self-Exchange Reaction by Constrained Density Functional Theory and Constrained Equilibrium Thermodynamics, J. Phys. Chem. A, 117 (33), 8017–8025. (2013).
- -Caetano,M.S.; Ramalho, T.C.; Botrel, D.F.; da Cunha, E.F.F.; Mello, W.C.; Understanding the Inactivation Process of Organophosphorus Herbicides: A DFT Study of Glyphosate Metallic Complexes with Zn2+, Ca2+, Mg2+, Cu2+, Co3+, Fe3+, Cr3+, and Al3+, International Journal of Quantum Chemistry, 112, 2752–2762, (2012).
- -Koo, I.N.; Ali, D.; Yang, K.; Park, Y.; Wardlaw, D.M.; Buncel, E.; Theoretical Study of 31P NMR Chemical Shifts for Organophosphorus Esters, Their Anions and O,O-Dimethylthiophosphorate Anion with Metal Complexes, Bull. Korean Chem. Soc., 29, 11, 2252-2258, (2008).
- -Amsterdam Density Functional (ADF) Code, Release, Vrije Universiteit, Amsterdam, The Netherlands, 2007.
- -E. Van Lenthe, E.J. Baerends, G. Snijders, Relativistic total energy using regular approximations, J. Chem. Phys., 101, 9783, (1994).
- -G. Te Velde, F.M. Bickelhaupt, S.J.A. Van Gisberger, C. Fonseca Guerra, E.J. Baerends, J.G. Snijders, Chemistry with ADF , T.J. Ziegler, Comput. Chem., 22, 931, (2001).
- -L. Verluis, T. Ziegler, The determination of molecular structures by density functional theory. The evaluation of analytical energy gradients by numerical integration, J. Chem. Phys., 88, 322, (1988).
- -S.H. Vosko, L. Wilk, M. Nusair, Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis, Can. J. Phys., 58, 1200, (1980).
- -P.R.T. Schipper, O.V. Gritsenko, S.J.A. van Gisbergen, E.J. Baerends, Molecular calculations of excitation energies and (hyper)polarizabilities with a statistical average of orbital model exchange-correlation potentials, J. Chem. Phys., 112, 1344, (2000).
- -E. Runge, E.K.U. Gross, Density-Functional Theory for Time-Dependent Systems, Phys. Rev. Lett., 52, 997, (1984).
- -F. Wang, T. Ziegler, E. van Lenthe, S. van Gisbergen, E.J. Baerends, The calculation of excitation energies based on the relativistic two-component zeroth-order regular approximation and time-dependent density-functional with full use of symmetry , J. Chem. Phys., 122, 204103, (2005).
- -A. Klamt, V. Jonas, A Conductor-like screening model for real solvents: A new approach to the quantitative calculation of salvation phenomenaJ. Chem. Phys., 105, 9972, (1996).
- -A. Klamt, Treatment of the outlying charge in continuum salvation models, J. Phys. Chem., 99, 2224, (1995).
- -N.M. O’Boyle, A.L. Tenderholt, K.M. Langner, Cclib: A library for package‐independent computational chemistry algorithms, J. Comput. Chem., 29, 839, (2008).
- -Anita Drozdz, Martina Bubrin, Jan Fiedler, Stanislav Z´aliˇs and Wolfgang Kaim, (a-Diimine) tricarbonylhalorhenium complexes: the oxidation side. Dalton Trans., 41, 1013, (2012).
- -F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen. Tables of bond Lengths determined by X-Ray and Neutron Diffraction. Part 1. Bond Lengths in Organic Compounds. J. Chem. Soc. Perkin Trans. II, S1-S19, (1987).
- -Andrzej Okuniewski and Barbara Becker, Ammonium O, O0-diethyl dithiophosphate, Acta Cryst., E67, o1749–o1750, (2011).
- -Rindorf, G.; Carlsen, L. Acta Cryst., B35, 1179, (1979).
- -Kenneth B. Wiberg and Yigui Wang, A comparison of some properties of C=O and C=S bonds, ARKIVOC 2011 (v) 45-56.
- -Athanassios C. Tsipis, Exploring the Forces That Control the P-C Bond Length in Phosphamides and Their Complexes: The Key Role of Hyperconjugation, Organometallics, 25, 2774-2781, (2006).
- -Srinivasan Priya, Maravanji S. Balakrishna, Joel T. Mague, Shaikh M. Mobin, Insertion of Carbon Fragments into P(III)-N Bonds in Aminophosphines and Aminobis(phosphines): Synthesis, Reactivity, and Coordination Chemistry of Resulting Phosphine Oxide Derivatives. Crystal and Molecular Structures of (Ph2P(O)CH2)2NR (R ) Me, nPr, nBu), Ph2P(O)CH(OH)nPr, and cis-[MoO2Cl2{(Ph2P(O)CH2)2NEt- KO,KO}], Inorganic Chemistry, 42, 1272-1281, (2003).
- -Sebastian Burck, Dietrich Gudat, Kalle Nättinen, Martin Nieger, Mark Niemeyer, Dirk Schmid, 2-Chloro-1,3,2-diazaphospholenes – A Crystal Structural Study, Eur. J. Inorg. Chem., , 5112–5119, (2007).
- -Christoph E. Strasser, Stephanie Cronje and Helgard G. Raubenheimer, Acta Cryst., E65, m86. doi:10.1107/S1600536808041809, (2009).
- -Yan-Fei Zhang, Pei-Hua Zhao, Jun-Jie Liu and Gui-Zhe Zhao, Acta Cryst., E67, o2861. doi:10.1107/S160053681104030X, (2011).
- -R. Matters and L. Nieland, Inorganica Chimica Acta, 112 ,215-217,(1986).
- -Stewart W. Bartlett, Simon J. Coles, David B. Davies, Michael B. Hursthouse, Hanife I :bis¸ogˇlu, Adem Kilic¸ Robert A. Shawb and I: lker U¨ nc, Acta Cryst., B62, 321–329. doi:10.1107/S0108768106000851, (2006).
- -C. Akers, S.W. Peterson and R. D. Willett, Acta Cryst., B24, 1125,(1968).
- -Reeve, R. N. Aspects of the coordination chemistry of phosphorus(V) chloro-compounds, Durham theses, Durham University, 1975.
- -George Socrates, Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3th ed.; John Wiley & Sons, Ltd: Chichester, England, , p. 82-90, 2001.
- -(Shaoyi Jiang, Siddharth Dasgupta, Mario Blanco, Rawls Frazier, Elaine S. Yamaguchi, Yongchun Tang,| and William A. Goddard, ,†Structures, Vibrations, and Force Fields of Dithiophosphate Wear Inhibitors from ab Initio Quantum Chemistry, J. Phys. Chem., 100, 15760-15769,(1996).
- -D. H. Boal, G. A. Ozin, Studies of some thermally unstable complexes of group V trihalides with trimethylamine and [2H9]trimethylamine by infrared, Raman, and matrix-isolation Raman spectroscopy, normal co-ordinate analysis, and structural methods, J. Chem. Soc., Dalton Trans., 1824-1828,(1972).
- -Hajar Sahebalzamani, Shahriare Ghammamy, Kheyrollah Mehrani, Shahram Jahandide, Farshid Salimi, Density functional theory studies of structural properties, energies and natural band orbital for two new aluminate compounds, Spectrochimica Acta Part A 90, 218– 222,(2012).
- -K.A.E. Roberts, Neil J. Brown, Hannah N. Roberts, Joseph J.W. McDouall, Paul J. Low, Mark W. Whiteley, Electronic structure and spectroscopy of the cycloheptatrienyl molybdenum halide complexes [MoBrL2(g-C7H7)] n+ (L2 = 2CO, n = 0; L2 = 2,20-bipyridyl, n = 0 or 1), Polyhedron, in press (2014).
- -Reshak, A. H, Sikander Azam,. “Theoretical Study Of The Structural, Electronic Structure, Fermi Surface, Electronic Charge Density and Opti¬cal Properties of the of LnVO4(Ln= Sm, Eu, Gd and Dy)” Int. J. Electro¬chem. Sci., 8, 10396 – 10423, (2013).
- -P.P. Moorthi, S. Gunasekaran, S. Swaminathan, G.R. Ramkumaar, Quantum chemical density functional theory studies on the molecular structure and vibrational spectra of mannitol, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 137, 412–422,(2015).
- -Jursic, B.S. “A B3LYP hybrid density functional theory study of struc¬tural properties, energies, and heats of formation for silicon–hydroge compounds”Journal of Molecular Structure (Theochem) 497, 65–73, (2000).
- -V. Nagarajan, R. Chandiramouli, TeO2 nanostructures as a NO2 sensor: DFT investigation, Computational and Theoretical Chemistry. 1049, 20– 27, (2014).


