- Sorption,
- pesticides,
- cyclic phosphazene
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
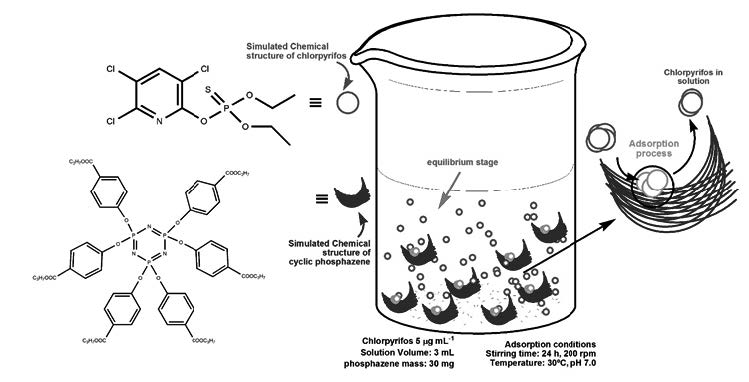
The effects of temperature, pH and agitation time (equilibrium) on the adsorption process of different pesticides on N3P3(OC6H4COOCH2CH2CH3)6 was studied. With optimal conditions experimental, the adsorption isotherms have been realized with through Langmuir and Freundlich models. Pesticides are compounds used mainly in agriculture to control various species (plants, insects, worms, fungi). Due to their physicochemical properties, they can remain for a long time in the application sites, bioaccumulating and moving between environmental compartments which generate various environmental problems. The results obtained showed a physisorption mechanism for the five pesticides studied, with higher sorption for: azinphos methyl (93,5 mg kg-1), carbaryl (290.5 mg kg-1) and carbofuran (580.5 mg kg-1) at 20 ° C, according to the models used.
References
- (1) A. Mkindi, N. Mpumi, Y. Tembo, P.C. Stevenson, P.A. Ndakidemi, K. Mtei, R. Machunda, S.R. Belmain Ind. Crop. Prod., article in press (2017).
- (2) C. Zhang, S. Guanming, J. Shen, R-f. Hu R-f J. Integr Agr. 14(9) 1903 (2015).
- (3) U. Hass, S. Christiansen, M. Axelstad, M. Scholze, J. Boberg Reprod. Toxicol. 72, 97 (2017).
- (4) N.I. Rousis, E. Zuccato, S. Castiglioni Environ. Int. 99, 213 (2017).
- (5) V. Yusa, M. Millet, C. Coscolla, M. Roca Anal. Chim. Acta 3, 15 (2015).
- (6) M.F.A. Jallow, D.G. Awadh, M.S. Albaho, V.Y. Devi, B.M. Thomas Environ. Int. 107, 100 (2017).
- (7) E. Silva, M.A. Daam, M.J. Cerejeira Chemosphere 135, 394 (2015).
- (8) J.P. Pascual Aguilar, V. Andreu, J. Campo, Y. Picó, A. Masiá Sci. Total Environ. 607–608, 752 (2017).
- (9) J.L. Sánchez-Osorio, J.V. Macías-Zamora, N. Ramírez-Álvarez, T.F. Bidleman Chemosphere, 173, 275 (2017).
- (10) LA. Suddaby, S. Beulke, W. van Beinum, R.G. Oliver, S. Kuet, C.D. Brown Chemosphere 162, 40 (2016).
- (11) Z. Zhao, Y. Jiang, Q. Li, Y. Cai, H. Yin, L. Zhang, J. Zhang Ecotoxicol. Environ. Safe. 142, 117 (2017).
- (12) V.D. Dang, K.J. Kroll, S.D. Supowit, R.U. Halden, N.D. Denslow Environ. Poll. 216, 877 (2016).
- (13) K. Demeestere, J. Dewulf, B. De Witte, H. Van Langenhove J. Chromatogr. A, 1153, 130 (2007).
- (14) G. Bapat, C. Labade, A. Chaudhari, S. Zinjarde Chemosphere 166, 21 (2017).
- (15) P. Janoš, M. Kormunda, F. Novák, O. Životský, J. Fuitová, V. Pilařová Reac. Func. Pol. 73, 46 (2013).
- (16) L.M. Jantunen, P.A. Helm, J.J Ridal, T.F. Bidleman Atmos. Environ. 36, 8533 (2008).
- (17) N.I. Rousis, R. Bade, L. Biilsma, E. Zuccato, J.V. Sancho, F. Hernandez, S. Casrtiglioni Environ. Res. 156, 31 (2017).
- (18) A. Thomas, L. Toms, F.A. Harden, P. Hobson, N. White, K. Mengersen, J. Mueller Environ. Res., 154, 10 (2017).
- (19) A. Achour, A. Derouiche, B. Barhoumi, B. Kort, D. Cherif, S. Bouabdallah, M. Sakly, K.B. Rhouma, S. Touil, M.R. Driss, O. Tebourbi Environ. Res. 156, 635 (2017).
- (20) D. Baglio, D. Kotzias, B. Richter Larsen J. Chromatog. A 854 (1–2), 207 (1999).
- (21) Z. Cheng, F. Dong, J. Xu, Z. Liu, Z. Chen, X. Pan, J. Gan, Y. Zheng Food Chem. 231, 365 (2017).
- (22) F.J. Lara, D. Chan, M. Dickinson, A.S. Lloyd, S.J. Adams J. Chromatogr. A, 1496, 37 (2017).
- (23) A. Parvin Zohrabi, M. Shamsipur, M. Hashemi, B. Hashemi Talanta 160, 340 (2016).
- (24) M.H.B. Müller, A. Polder, O.B. Brynildsrud, M. Karimi, E. Lie, W.B. Manyilizu, R.H. Mdegela, F. Mokiti, M.M. Murtadha, H.E. Nonga, J.U. Skaare, J.L. Lyche Environ. Res. 154, 425 (2017).
- (25) W. Huang, P. Peng, Z. Yu, J. Fu Appl. Geochem. 18, 955 (2003).
- (26) F. Sanchez-Rojas, C. Bosch-Ojeda, J.M. Cano-Pavon Chromatographia 69, 79 (2009).
- (27) C.A. Oliveira Ribeiro, Y. Vollaire, A. Sanchez-Chardi, H. Roche Aquatic Toxicol. 74, 53 (2005).
- (28) N. Ochiaia, T. Ieda, K. Sasamoto, Y. Takazawa, S. Hashimoto, A. Fushimi, K. Tanabe J. Chromatogr. A 1218, 6851 (2011).
- (29) G. Satpathy, Y. Tyagi, R. Gupta Food Chem. 127, 1300 (2011).
- (30) M.R. Driss, M.C. Hennion, M.L. Bouguerra J. Chromatog. A 639, 352 (1993).
- (31) G. Ehlers, A. Loibner Environ. Poll. 141, 494 (2006).
- (32) H.R. Allcock Chem. Rev. 72, 315 (1972).
- (33) C.W. Allen Chem. Rev. 91, 119 (1991).
- (34) D. E. Çirali, O. Dayan, N. Özdemir, N. Hacıoglu Polyhedron 88, 170 (2015).
- (35) D. Kumar, A.J. Elias J. Fluor. Chem. 166, 69 (2014).
- (36) A. Uslu, E. Özcan E. J. Mol. Struc. 1142, 116 (2017).
- (37) I. Ün, H. İbişoğlu, S.S. Ün, B. Çoşut, A. Kiliç A. Inor. Chim. Acta 399, 219 (2013).
- (38) D. Prasanna, V. Selvaraj J. Coll. Int. Sci. 472, 116 (2016).
- (39) Y.J. Shin, Y.R. Ham, S.H. Kim, D.H. Lee, S.B. Kim, C.S. Park, Y.M. Yoo, J.G Kim, Kwon, S.H. Shin J. Ind. Eng. Chem. 16, 364 (2010).
- (40) N. Ochiai, K. Sasamoto, F. David, P. Sandra J. Chromatogr A, 1455, 45 (2016).
- (41) H. Piri-Moghadam, E. Gionfriddo, A. Rodriguez-Lafuente, J.J. Grandy, H.L. Lord, T. Obal, J. Pawliszyn Anal. Chim Acta 964, 74 (2017).
- (42) G. Bapat, C. Labade, A. Chaudhari, S. Zinjarde Adv. Coll. Inter. Sci. 237, 1 (2016).
- (43) H. Esfandian, A. Samadi-Maybodi., B. Khoshandam, M. Parvini J. Taiwan Inst. Chem. Eng. 75, 164 (2017).
- (44) S. Zhang, Q. Yang, X. Yang, W. Wang, Z. Li, L. Zhang, C. Wang, Z. Wang Talanta 166, 46 (2017).
- (45) A. Derylo-Marczewska, M. Blachnio, A.W. Marczewski, A. Swiatkowski, B. Buczek Chem. Eng. J. 308, 408 (2017).
- (46) P. Vanraes, H. Ghodbane, D. Davister, N. Wardenier, A. Nikiforov, Y.P. Verheust, Van S.W.H. Hulle, Q. Hamdaoui, J. Vandamme, J. Van Durme, P. Surmont, F. Lynen, C. Leys Water Res. 116, 1 (2017).
- (47) S. Hua, J.L. Gong, G-M Zeng, F-B Yao, M. Guo, X-M Ou Chemosphere 177, 65 (2017).
- (48) P.K. Boruah, B. Sharma, N. Hussain, M.R. Das Chemosphere 168, 1058 (2017).
- (49) Chen Z., Fu J., Wang M., Wang X., Hang R. J. Zhang, Q. Xu J. Hazard, Mater. 273, 263 (2014).
- (50) Z. Chen, J. Fu, M. Wang, X. Wang, J. Zhang, Q. Xu Appl. Surf. Sci. 289, 495 (2014).
- (51) M. Maeda, K. Kuroyanagi, S. Sakurai, T. Yamanaka, E. Yashima Macromolecules 44 (8), 2457 (2011).
- (52) A. Welle, M. Grunze, D. Tur J. Colloid Interf. Sci. 197, 263 (1998).
- (53) K. Miyata, Y. Watanabe, T. Itaya, T. Tanigaki, K. Inoue Macromolecules 29, 3694 (1996).
- (54) W. Zheng, M. Guo, T. Chow, D. Bennett, N. Rajogopalan J. Hazard. Mater. 181, 121 (2010).
- (55) J. Rivas, M.I. Toral., P. Richter J. Chil. Chem. Soc. 57, 1087 (2012).
- (56) Y. Qiu, X. Xiao, H. Cheng, Z. Zhou, G.D. Sheng Environ. Sci. Technol. 43, 4973 (2009).
- (57) Y. He, J. Xu, H. Wang, Z. Ma, J. Chen Environ. Res. 101, 362 (2006).
- (58) G. Limousin, J.P. Gaudet, L. Charlet, S. Szenknect, V. Barthes, M. Krimissa App. Geochem. 22, 249 (2007).
- (59) S. Salvestrini, V. Leone P. Iovino, S. Canzano, S. Capasso J. Chem. Thermodyn. 68, 310 (2014).


