NANODECORATION OF SINGLE CRYSTALS OF 5,11,17,23-TETRA-TERT-BUTYL-25,27- BIS(CYANOMETHOXY)-26,28-DIHYDROXYCALIX[4]ARENE
- Single crystals,
- 1,
- 3-bis(cyanomeththoxy)calix[4]arene,
- nanodecorated,
- Gold Nanoparticles
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
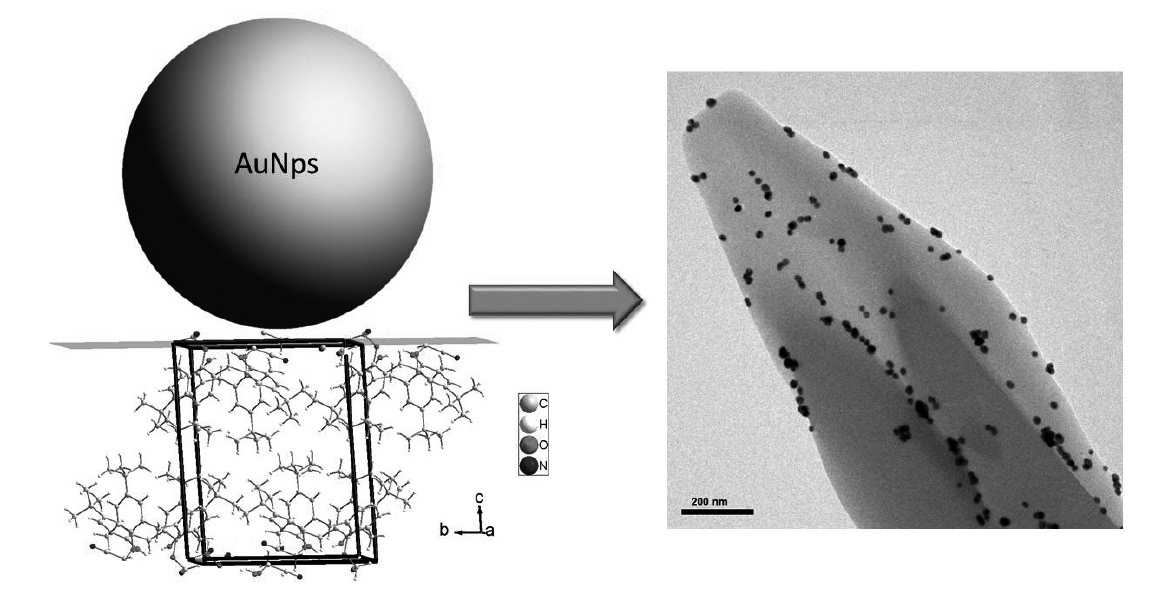
Single crystals of 1,3-bis(cyanomethoxy)calix[4]arene (1) were grown from Chloroform/Methanol mixture and these were nanodecorated with colloidal gold nanoparticles (AuNps). The single crystals were then characterized by single-crystal X-ray diffraction (XRD) and Scanning Electronic Microscopy (SEM). The nanodecorated crystals were characterized by UV-Visible Absorption Spectroscopy, Transmission Electronic Microscopy (TEM) and X-ray Photoelectron Spectroscopy (XPS). In this work, it is shown that the stabilization of the nanoparticles occurs through of the interactions of these with the nitrile group, maintaining their shape and size.
References
- (1) Iqbal, M. Gutsche, C. D. Org. Synth. 1993, 8, 100–102.
- (2) Zhang, Wen-Chun, Huang, Z.-T. Synthesis (Stuttg). 1997, 9, 1073–1076.
- (3) Asfari, M.-Z., Böhmer, V., Harrowfield, J., Vicens, J. Calixarenes 2001; Springer: Berlin , Germany, 2001.
- (4) Predeus, A. V; Gopalsamuthiram, V.; Staples, R. J.; Wulff, W. D. Angew. Chem.Int. Ed. Engl. 2013, 52 (3), 911–915.
- (5) Baldini, L.; Casnati, A.; Sansone, F. In Comprehensive Supramolecular Chemistry {II}; Atwood, J. L., Ed.; Elsevier: Oxford, 2017; pp 371–408.
- (6) Ukhatskaya, E. V; Kurkov, S. V; Matthews, S. E.; El Fagui, A.; Amiel, C.; Dalmas, F.; Loftsson, T. Int. J. Pharm . 2010, 402 (1–2), 10–19.
- (7) Grives, S.; Phan, G.; Bouvier-Capely, C.; Suhard, D.; Rebiere, F.; Agarande, M.; Fattal, E. Chem. Biol. Interact. 2017, 267, 33–39.
- (8) Zhao, B.; Liu, Y.; Zhang, H. J. Mol. Struct. 2004, 691, 25–31.
- (9) Moris, S.; Galdámez, A.; Jara, P.; Saitz-barria, C. Crystals 2016, 6 (114).
- (10) Perrin, M.; Ehlinger, N.; Lecocq, S.; Dumazet, I.; Lamartine, R. J. Incl. Phenom.Macrocycl. Chem. 2001, 82282 (82282), 273–276.
- (11) Mutihac, L.; Lee, J. H.; Kim, J. S.; Vicens, J. Chem Soc rev 2011, 40 (5), 2777–2796.
- (12) M. Anthony McKervey, E. M. S. J. Org. Chem. 1986, 51, 3581–3584.
- (13) Huang, G.; Jiang, L.; Wang, D.; Chen, J.; Li, Z.; Ma, S. J. Mol. Liq. 2016, 220, 346–353.
- (14) C.Quiroga-Campano, H.Gomez-Machuca, S.Moris, P. Jara, J.R. de la Fuente, H. Pessoa-Mahana, C. Jullian, C. S. J. Mol. Struct. 2017, 1141, 133–141.
- (15) Collins, E., Harrisc, S. J.; Owens, M.; Ferguson, G.; Estate, I.; M. Anthony McKervey, E. M. S. J. Chem. Soc. Perkin trans 1991, 3, 2–3.
- (16) Zhao, B.-T.; Liu, Y.; Zhang, H.-Y. J. Mol. Struct. 2004, 691 (1–3), 25–31.
- (17) Rodríquez-Llamazares, S.; Jara, P.; Yutronic, N.; Noyong, M.; Bretschneider, J.; Simon, U. J. Colloid Interface Sci 2007, 316 (1), 202– 205.
- (18) Li, H.; Yang, Y.-W. Chinese Chem. Lett. 2013, 24 (7), 545–552.
- (19) Arduini, A.; Demuru, D.; Pochini, A.; Secchi, A. Chem. Commun. 2005, No. 5, 645–647.
- (20) Sayin, S.; Ozcan, F.; Yilmaz, M. Mater. Sci. Eng. C 2013, 33 (4), 2433– 2439.
- (21) Dementjev, A. P.; Graaf, A. De; Sanden, M. C. M. Van De; Maslakov, K. I. Diam. Relat. Mater 2000, 9, 1904–1907.
- (22) Turkevich, J.; Stevenson, P. C.; Hillier, J. Discuss. Faraday Soc. 1951, 11 (0), 55–75.
- (23) Lévy, R.; Thanh, N. T. K.; Doty, R. C.; Hussain, I.; Nichols, R. J.; Schiffrin, D. J.; Brust, M.; Fernig, D. G. J. Am. Chem.Soc 2004, 126 (32), 10076–10084.
- (24) Silva, N.; Moris, S.; Díaz, M.; Yutronic, N.; Lang, E.; Chornik, B.; Kogan, M. J.; Jara, P. Molecules 2016, 21, 1–13.
- (25) Bruker SMART, SAINTPLUS V6.02, S. V. 1. and S. B. A. X. I. I.
- (26) Sheldrick, G.M. SHELXL-97. Program for the Refinement of Crystal Structures; University of Göttingen: Stuttgart, G. 1997.
- (27) Pennington W. J. Appl. Crystallogr. 1999, 32 (5), 1028–1029.
- (28) Speck, A. L. J. Appl. Crystallogr. 2003, 36, 7–13.
- (29) Dieluweit, S.; Pum, D.; Sleytr, U. B.; Kautek, W. Mat. Sci. Eng. 2005, 25, 727–732.
- (30) Zhou, J. C.; Wang, X.; Xue, M.; Xu, Z.; Hamasaki, T.; Yang, Y.; Wang, K.; Dunn, B. Mat. Sci. Eng. C 2010, 30 (1), 20–26.
- (31) Tseng, R. J.; Tsai, C.; Ma, L.; Ouyang, J.; Ozkan, C. S.; Yang, Y. Nat. Nanotechnol. 2006, 1 (October), 4–9.
- (32) Kimura-suda, H.; Petrovykh, D. Y.; Tarlov, M. J.; Whitman, L. J. J. Am. Chem.Soc 2003, 125, 9014–9015.


