A PRELIMINARY STUDY ON ELECTROCATALYTIC REDUCTION OF CO2 USING FAC-ReI(CO)3(4,4’-DIMETHYL- 2,2’-BIPYRIDYL)((E)-2-((3-AMINO-PYRIDIN-4-YLIMINO)-METHYL)-4,6-DI-TERT-BUTYLPHENOL))+ COMPLEX
- Rhenium tricarbonyl complexes,
- reduction carbon dioxide,
- vitreous carbon,
- electrochemistry
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
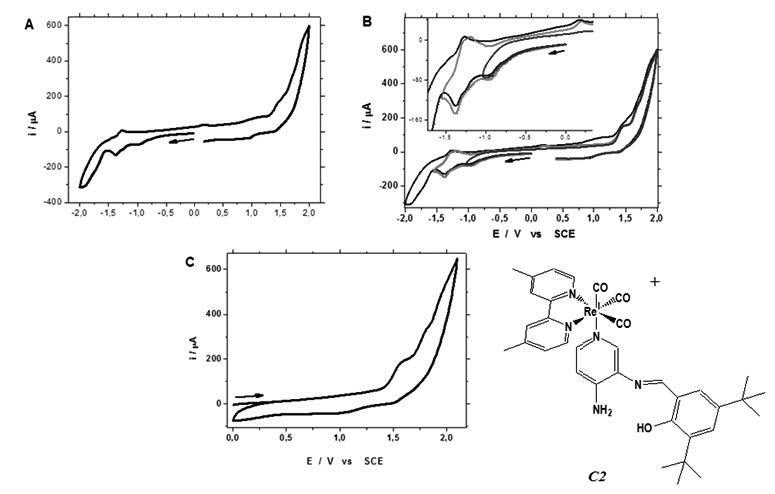
Several research to explore the possible conversion of CO2 using rhenium(I) tricarbonyl complexes have been reported the last years. In the present work, we investigated a potential use of fac-Re(CO)3(4,4’-di-methyl-2,2’-bipyridyl)L+ complex (C2), where L is an electron-withdrawing ancillary ligands which present an intramolecular hydrogen bond (IHB), in a preliminary electrocatalytic reduction of CO2. The C2 complex was synthesized and characterized according to reported methods earlier. The cyclic voltammogram profile for the C2 complex were studied in dichloromethane under inert atmosphere, and it shows a typical behavior for an electrocatalytic process, the C2 complex illustrate the electrochemical reaction mechanism corresponds to an electrochemical–chemical–electrochemical pathway (ECE). Also, a Vitreous Carbon plate used as working electrode was employed and modified by cycling the anodic region of C2 in CH2Cl2 which involve the oxidative redox response for the -NH2 and -OH groups. The voltammogram profile involve shows a polymeric deposit on the plate surface in a CO2 saturated solution (pH=7.0). A strong electrocatalytic discharge of current is obtained with a wave foot of -1.3 V showing that C2 have the potential to be used in electrocatalyst CO2 reduction.
References
- J.L. Wedding , H.H. Harris, C.A. Bader, S.E. Plush, R. Mak, M. Massi, D.A. Brooks, B. Lai B., S. Vogt, M.V. Werrett, P.V. Simpson, B.W. Skelton, S. Stagni, Metallomics., 9, 382, (2017).
- S.T. Lam, N. Zhu, V.K.M. Au, V. Wing-Wah Yam, Polyhedron, 86, 10, (2015).
- J.M. Villegas, S.R. Stoyanov, W. Huang, D.P. Rillema, Inorg. Chem., 44, 2297, (2005).
- L. Wallace, D.P. Rillema, Inorg. Chem., 32, 3836, (1993).
- L.N. Winslow, D.P. Rillema, J.H. Welch, P. Singh, Inorg. Chem., 28, 1596, (1989).
- M.S. Wrighton, D.L. Morse, L. Pdungsap, J. Am. Chem. Soc., 8, 2073, (1975).
- M.P. Coogan, Platts J.A., Chem. Commun., 52, 12498, (2016).
- M. Kaplanis, G. Stamatakis, V.D. Papakonstantinou, M. Paravatou- Petsotas, C.A. Demopoulos, C.A. Mitsopoulou, Journal of Inorganic Biochemistry, 135, 1, (2014).
- T.Y. Kim, A.B.S. Elliott, K.J. Shaffer, C.J. McAdam, K.C. Gordon, J.D. Crowley, Polyhedron, 52, 1391, (2013).
- A. Carreño, M. Gacitua, E. Schott, X. Zarate, J.M. Manriquez, M. Preite, S. Ladeira, A. Castel, N. Pizarro, A. Vega, I. Chavez, R. Arratia-Perez, New J. Chem., 39, 5725, (2015).
- L.A. Sacksteder, A.P. Zipp, E.A. Brown, J. Streich, J.N. Demas, B.A. DeGraf, Inorg. Chem., 29, 4335, (1990).
- B. Machura, M. Wolff, E. Benoist, Y. Coulais, Journal of Organometallic Chemistry, 724, 82, (2013).
- G.M. Hasselmann, G.J. Meyer, The Journal of Physical Chemistry B, 103, 7671, (1999).
- M.K. Bera, C. Chakraborty, S. Malik, New J. Chem., 40, 8074, (2016).
- A. Carreño, M. Gacitúa, D. Páez-Hernández, R. Polanco, M. Preite, J.A. Fuentes, G.C. Mora, I. Chávez, R. Arratia-Pérez, New. J. Chem., 39, 7822, (2015).
- S.A. Chabolla, E.A. Dellamary, C.W. Machan, F.A. Tezcan, C.P. Kubiak, Inorganica Chimica Acta, 422, 109, (2014).
- A. Carreño, M. Gacitúa, J.A. Fuentes, D. Páez-Hernández, J.P. Peñaloza, C. Otero, M. Preite, E. Molins, W.B. Swords, G.J. Meyer, J.M. Manríquez, R. Polanco, I. Chávez, R. Arratia-Pérez, New J. Chem., 40, 7687, (2016).
- A. Carreño, A.A. Aros, C. Otero, R. Polanco, M. Gacitua, R. Arratia- Perez, J.A. Fuentes, New J. Chem., 41, 2140, (2017).
- G. Balakrishnan, T. Rajendran, K.S. Murugan, M.S. Kumar, V.K. Sivasubramanian, M. Ganesan, A. Mahesh, T. Thirunalasundari, S. Rajagopal, Inorganica Chimica Acta, 434, 51, (2015).
- F.L. Thorp-Greenwood, Organometallics, 31, 5686, (2012).
- W.H. Wang, Y. Himeda, J.T. Muckerman, G.F. Manbeck, E. Fujita, Chem. Rev., 115, 12936, (2015).
- T.A. Manes T.A., M.J. Rose, Inorganic Chemistry Communications, 61, 221, (2015).
- K. Parimal K., S. Vyas S., C.H. Chen, C.M. Hadad, A.H. Flood, Inorganica Chimica Acta, 374, 620, (2011).
- Y. Kuninobu, K. Takai K, Chem. Rev., 111, 1938, (2011).
- C.W. Machan, S.A. Chabolla, C.P. Kubiak, Organometallics, 34, 4678, (2015).
- J. Hawecker, J.M. Lehn, R. Ziezzel, J. Chem. Soc., Chemm. Commun., 536, (1983).
- J. Hawecker, J.M. Lehn, R.J. Ziezzel, J. Chem. Soc., Chemm. Commun., 6, 328, (1984).
- J. Hawecker, J.M. Lehn, R.J. Ziezzel, Helv. Chim. Acta, 69, 1990, (1986).
- J.M. Smieja, C.P. Kubiak, Inorg. Chem., 49, 9283, (2010).
- G. Valenti, M. Panigati, A. Boni, G. D’Alfonso, F. Paolucci, L. Prodi, Inorganica Chimica Acta, 417, 270, (2014).
- M.V. Vollmer, C.W. Machan, M.L. Clark, W.E. Antholine, J. Agarwal, H.F. Schaefer III, C.P. Kubiak, J.R. Walensky, Organometallics, 34, 3, (2015).
- C. Ko, A.W.Y Cheung., S.M. Yiu, Polyhedron, 86, 17, (2015).
- A. W. Kleij, M. Kuil, D. M. Tooke, M. Lutz, A. L. Spek, J. N. H. Reek, Chem. Eur. J., 11, 4743, (2005).
- A. Carreño, A. Vega, X. Zarate, E. Schott, M. Gacitua, N. Valenzuela, M. Preite, J.M. Manriquez, I. Chavez, Quim. Nova, 37, 584, (2014).
- A. Carreño, M. Gacitua, E. Schott, X. Zarate, J.M. Manriquez, M. Preite, S. Ladeira, A. Castel, N. Pizarro, A. Vega, I. Chavez, R. Arratia-Perez, New J. Chem, 39, 5725, (2015).
- G.F. Manbeck, J.T. Muckerman, D.J. Szalda, Y. Himeda, E. Fujita, J. Phys. Chem. B, 24, 7457, (2015).
- A. Carreño, M. Gacitua, E. Molins, R. Arratia-Perez, Chem. Pap. DOI 10.1007/s11696-017-0196-6, (2017).
- R. Czerwieniec, A. Kapturkiewicz, J. Lipkowski, J. Nowacki, Inorg. Chim. Acta, 358, 2701, (2005).
- G.F. Manbeck, J.T. Muckerman, D.J. Szalda, Y. Himeda, E. Fujita, J. Phys. Chem. B, 24, 7457, (2015).
- W. Herzog, C. Bronner, S. Loffler, B. He, D. Kratzert, D. Stalke, A. Hauser, O.S. Wenger, Chem. Phys. Chem., 14, 1168, (2013).
- R. Lopez, B. Loeb, D. Striplin, M. Devenney, K. Omberg, T.J. Meyer, J. Chil. Chem. Soc., 49, 149, (2004).
- M. Liang, W. Xu, F. Cai, P. Chen, B. Peng, J. Chen, Z. Li, J. Phys. Chem. C., 111, 4465, (2007).
- W. Xu, B. Peng, J. Chen, M. Liang, F. Cai, J. Phys. Chem. C, 112, 874, (2008).
- M.W. Louie, H.W. Liu, M.H.C. Lam, T.C. Lau, K.K.W. Lo, Organometallics, 28, 4297, (2009).
- C. Bruckmeier, M.W. Lehenmeier, R. Reithmeier, B. Rieger, J. Herranz, C. Kavakli, Dalton Trans., 41, 5026, (2012).
- A. W. Kleij, D.M. Tooke, A.L. Spek, J.N.H. Reek, Eur. J. Inorg. Chem., 4626, (2005).
- S. Sato, T. Morimoto, O. Ishitani, Inorg. Chem., 46, 9051, (2007).
- A. Blake, N. Champness, T. Easun, D. Allan, H. Nowel, M. George, J. Sun, Nat. Chem., 2, 688, (2010).
- C.W. Machan, M.D. Sampson, S.A. Chabolla, T. Dang, C.P. Kubiak, Organometallics, 33, 4550, (2014).
- M.T. Zhang, T. Irebo, O. Johansson, L. Hammarstrom, J. Am. Chem. Soc., 133, 13224, (2011).
- J. Agarwal, E. Fujita, H.F. Schaefer, J.T. Muckerman, J. Am. Chem. Soc., 134, 5180, (2012).
- Q. Zeng, M. Messaoudani, A. Vlcek Jr., F. Hartl, Eur. J. Inorg. Chem., 471, (2012).
- P.I. Nagy, Int. J. Mol. Sci., 15, 19562, (2014).
- H.C. Bertrand, S. Clede, R. Guillot, F. Lambert, C. Policar, Inorg. Chem. 53, 6204, (2014).
- B. Machura, M. Wolff, E. Benoist, Inorganic Chemistry Communications, 29, 101, (2013).
- C. Costentin, G. Passard, M. Robert, J.M. Saveant, PNAS, 42, 14990, (2014).
- O.S. Wenger, Coord. Chem. Rev., 282, 150, (2015).
- J. Sanchez, B.L. Rivas, J.C. Moutet, D.P. Oyarzun, J. Chil. Chem. Soc., 61, 3277, (2016).
- M.A. Riquelme, M. Isaacs, M. Lucero, E. Trollund, M.J. Aguirre, J. Canales, J. Chil. Chem. Soc., 48, 89, (2003).
- M. del Valle, Y. Chen, A. Alarcon, A. Ramos, L. Hernandez, C. Canales, B. Gonzalez, J. Chil. Chem. Soc., 58, 1991, (2013).
- A. Carreño, E. Solís-Céspedes, D. Paez-Hernandez, R. Arratia-Perez, Chem. Phys. Letter, 685, 354 (2017).
- M. Isaacs, J.C. Canales, A. Riquelme, M. Lucero, M.J. Aguirre, J. Costamagna, Journal of Coordination Chemistry, 56, 1193, (2003).
- C. Costentin, J. C. Canales, B. Haddou, J.M. Saveant, J. Am. Chem. Soc., 135, 17671, (2013).


