Vol 62 No 4 (2017): Journal of the Chilean Chemical Society
Original Research Papers
ACTIVITY OF ALUMINA SUPPORTED Fe CATALYSTS FOR N2O DECOMPOSITION: EFFECTS OF THE IRON CONTENT AND THERMAL TREATMENT
Published
February 9, 2018
Keywords
- iron,
- alumina,
- impregnation method,
- N2O decomposition
How to Cite
Alvarez, P., Araya, P., Rojas, R., Guerrero, S., & Aguila, G. (2018). ACTIVITY OF ALUMINA SUPPORTED Fe CATALYSTS FOR N2O DECOMPOSITION: EFFECTS OF THE IRON CONTENT AND THERMAL TREATMENT. Journal of the Chilean Chemical Society, 62(4). Retrieved from https://jcchems.com/index.php/JCCHEMS/article/view/473
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
The activity of Fe2O3/Al2O3 catalysts prepared by impregnation of Al2O3 with different amounts of Fe and calcination temperatures (650 and 900 ºC) in the direct N2O decomposition reaction was studied. High calcination temperature was introduced to study the effect of “aging”, which are the conditions prevailing in the process-gas option for N2O abatement. The catalysts were characterized by BET, XRD, UV-DRS, and H2-TPR. The incorporation of Fe promotes the alumina phase transition (g-Al2O3 to a-Al2O3) when the catalysts are calcined at 900 ºC, which is accompanied by a decrease in the specific area. The activity of the catalysts and the specific surface area depend on Fe loading and calcination temperature. It was found that highly dispersed Fe species are more active than bulk type Fe2O3 particles. We conclude that Fe2O3/Al2O3 catalysts prepared by impregnation method are active in the decomposition of N2O, to be used at low or high reaction temperatures (tail-gas or process-gas treatments, respectively), as part of nitric acid production plant.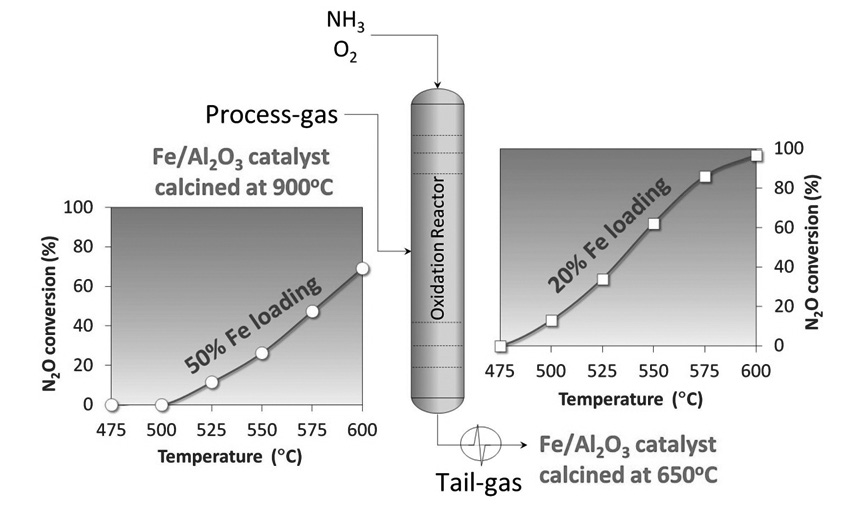
References
- G. Centi, S. Perathoner, F. Vanazza, M. Marella, M. Tomaselli, M. Mantegazza, Adv. Environ. Res. 4 (2000) 325-338.
- M. Hevia, J. Pérez-Ramírez, Applied Catalysis B 77 (2008) 248-254.
- E. Malki, R. van Santen, W. Sachtler, J. Catal. 196 (2000) 212-223.
- B. Wood. J. Reimer, A. Bell, M. Janicke, K. Ott, J. Catal. 224 (2004) 148- 155.
- K. Sun, H. Xia, E. Hensen, R. van Santen, C. Li, J. Catal. 238 (2006) 186- 195.
- G. Pirngruber, P. Roy, R. Prins, J. Catal. 246 (2007) 147-157.
- L. Pirutko, V. Chernyavsky, E. Starokon, A. Ivanov, A. Kharitonov, G. Panov, Applied Catalysis B 91 (2009) 174-179.
- M. Rivallan, G. Ricchiardi, S. Bordiga, A. Zecchina, J. Catal. 264 (2009) 104-116.
- P. Sazama, N. Sathu, E. Tabor, B. Wichterlova, S. Sklenak, Z. Sobalik, J. Catal. 299 (2013) 188-203.
- G. Panov, V. Sobolev, A. Kharitonov, J. Mol. Catalysis 61 (1990) 85-97.
- G. Pirngruber, M. Luechinger, P. Roy, A. Cecchetto, P. Smirniotis, J. Catal. 224 (2004) 429-440.
- I. Yuranov, D. Bulushev, A. Renken, L. Kiwi-Minsker, J. Catal. 227 (2004) 138-147.
- E. Kondratenko, J. Pérez-Ramírez, Journal of Physical Chemistry B 110 (2006) 22586-22595.
- A. Heyden, B. Peters, A. Bell, F. Keil, Journal of Physical Chemistry B 109 (2005) 1857-1873.
- G. Giecko, T. Borowiecki, W. Gac, J. Kruk, Catalysis Today 137 (2008) 403-409.
- J. Kruk, K. Stolecki, K. Michalska, M. Konkol, P. Kowalik, Catalysis Today 191 (2012) 125-128.
- P. Pomonis, D. Vattis, A. Lycourghiotis, C. Kordulis, J. Chem. Soc. Faraday Trans. 1, 81 (1985) 2043-2051.
- C. Kordulis, H. Latsios, A. Lycourghiotis, P. Pomonis, J. Che. Soc. Faraday Trans. 86 (1990) 185-187.
- S. Christoforou, E. Efthimiadis, I. Vasalos, Catalysis Letters 79 (2002) 137-147.
- G. Pekridis, C. Athanasiou, M. Konsolakis, I. Yentekakis, G. Marnellos, Top. Catal. 52 (2009) 1880-1887.
- M. Colaianni, P. Chen, J. Yates, Surface Science 238 (1990) 13-24.
- Z. Zhong, T. Prozorov, I. Felner, A. Gedanken, Journal of Phys. Chem. B 103 (1999) 947-956.
- Z. Li, J. Sheng, Y. Wang, Y. Xu, Journal of Hazardous Materials 254-255 (2013) 18-25.
- J. Pérez-Ramírez, M. Kumar, A. Bruckner, Journal of Catalysis 223 (2004) 13-27.
- E. Berrier, O. Ovsitser, E. Kondratenko, M. Schwidder, W. Grunert, A. Bruckner, Journal of Catalysis 249 (2007) 67-78.
- A. Koekkoek, W. Kim, V. Degirmenci, H. Xin, R. Ryoo, E. Hensen, Journal of Catalysis 299 (2013) 81-89.
- M. Nechita, G. Berlier, G. Martra, S. Coluccia, F. Arena, G. Italiano, G. Trunfio, A. Parmaliana, Il Nuovo Cimento B 123 (2008) 1541-1551.
- M. Tepluchin, D. Pham, M. Casapu, L. Madler, S. Kureti, J. Grunwaldt, Catal. Sci. Technol. 5 (2015) 455-464.
- O. Wimmers, P. Arnoldy, J. Moulijn, Journal of Physical Chemistry 90 (1986) 1331-1337.
- M. Tiernan, P. Barnes, G. Parkes, Journal of Physical Chemistry B 105 (2001) 220-228.
- A. Venugopal, J. Aluha, D. Mogano, M. Scurrell, Applied Catalysis A 245 (2003) 149-158.
- W. Jozwiak, E. Kaczmarek, T. Maniecki, W. Ignaczak, W. Maniukiewicz, Applied Catalysis A 326 (2007) 17-27.
- J. Zielinski, I. Zglinicka, L. Znak, Z. Kaszkur, Applied Catalysis A 381 (2010) 191-196.
- P. Michorczyk, P. Kustrowski, L. Chmielarz, J. Ogonowski, Reaction Kinetics Catalysis Letters 82 (2004) 121-130.
- H. Zhou, Y. Su, W. Liao, W. Deng, F. Zhong, Applied Catalysis A 505 (2015) 402-409.
- J. Peréz-Ramírez, F. Kapteijn, A. Bruckner, Journal of Catalysis 218 (2003) 234-238.
- T. Nobukawa, M. Yoshida, K. Okumura, K. Tomishige, K. Kunimori, Journal of Catalysis 229 (2005) 374-388.
- P. Sazama, B. Wichterlova, E. Tabor, P. Stastny, N. Sathu, Z. Sobalik, J. Dedecek, S. Sklenak, P. Klein, A. Vondrova, Journal of Catalysis 312 (2014) 123-138.
- J. Wang, H. Xia, X. Ju, Z. Feng, F. Fan, C. Li, Journal of Catalysis 300 (2013) 251-259.
- T. Vulic, A. Reitzmann, K. Lazar, Chemical Engineering Journal 207-208 (2012) 913-922.
- G. Moretti, G. Fierro, G. Ferraris, G. Andreozzi, V. Naticchioni, Journal of Catalysis 318 (2014) 1-13.


