DEGRADATION OF POLY(ETHYLENETEREPHTALATE) WASTE TO OBTAIN OLIGOMERS USING A ZINC COMPLEX AS CATALYST
- Poly(ethyleneterephthalate),
- degradation,
- catalyst,
- chemical recycling,
- glycolysis
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
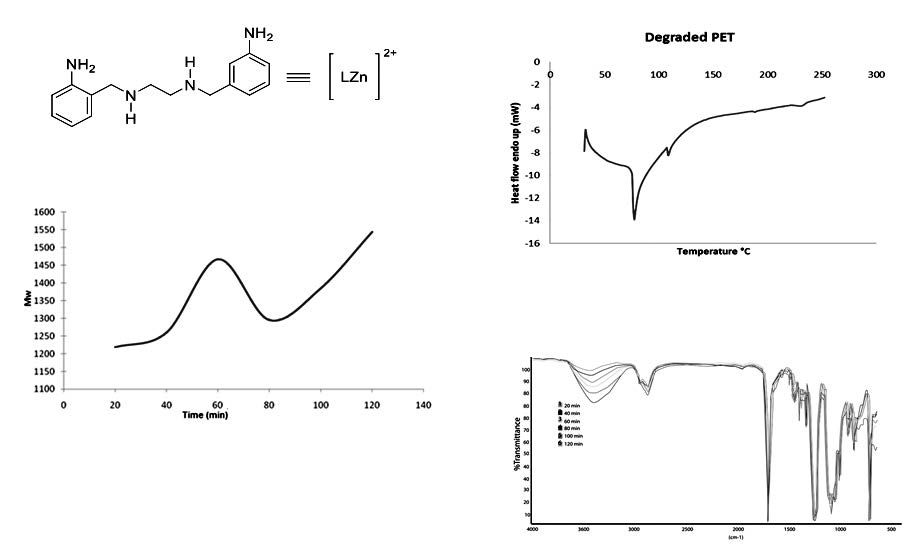
Thermal degradation of Poly(ethylene terephthalate) (PET) bottles waste was carried out by glycolysis using triethyleneglycol (TEG) as nucleophilic agent and N1,N2-bis(2-aminobenzyl)-1,2-diaminoethane zinc(II) (ABEN), in order to test this compound as catalyst in the degradation of PET until to obtain short chains of polyester (oligomers). The depolymerization processes was tested at 180, 190 and 210 °C, and two molar ratios of PET:TEG (1:1.3 and 1:2.6), the amount of catalyst used was fixed at 1% in relation to PET, the best experiment of PET glycolysis was found at 190 °C during two hours in a mass relation PET:TEG 1:1.3. All products were analyzed by Fourier transform infrared spectroscopy (IR), gel permeation chromatography (GPC) and differential scanning calorimetry (DSC), the behavior of the reaction rate was studied for the best experiment by measuring the amount of degraded PET at different glycolysis time. The degradation of PET was favored with the use of ABEN, which accelerates the reaction, obtaining oligomers in the absence of an organic solvent or reaction medium, this is the most efficient catalyst found so far. The oligomers were obtained with molecular weight between 900 and 1500 g mol-1 depending on the reaction time, 60 min was the less time of the total glycolysis of PET. Also was found that the molecular weight of the oligomers increased along the glycolysis, due to the recombination with TEG and ethylene glycol produced in degradation.
References
- S.M. Cakić, I.S. Ristić, M. M-Cincovik, N.C. Nikolić, O.Z. Ilić, D.T. Stojiljković, J.K. B-Simendić, Prog. Org. Coat. 74, 124, (2012).
- C. Gioia, M. Vannini, P. Marchese, A. Minesso, R. Cavalieri, M. Colonna, A. Celli, Green Chem. 16, 1808, (2014).
- A. El-Mejjatti, T. Harit, A. Riahi, R. Khiari, I. Bouabdallah, F. Malek, Polymer Letters 8, 544, (2014).
- A.P. More, R.A. Kute, Iran. Polym. J. 23, 59, (2014).
- M.E. Tawfik, S.B. Eskander, Polym. Degrad. Stabil. 95, 187, (2010).
- R.M. Musale, S.R. Shukla, Int. J. Plast. Technol. 20, 106, (2016).
- A. Aguado, L. Martínez, L. Becerra, M. Arieta-Araunabeña, S. Arnaiz, A. Asueta, I. Robertson, J. Mater. Cycles Waste 16, 201, (2014).
- M.F.A. Viante, C.S. Nunes, E.C. Muniz, M.L. Felsner, C.A.P. Almeida, Polym. Int. 65, 1024, (2016).
- A.M. Al-Sabagh, F.Z. Yehia, Gh. Eshaq, A.M. Rabie, A.E. ElMetwally, Egyptian J. Of Petroleum. 25, 53, (2016).
- A. Ptičck, A. Fijačko, Z. Hrnjak-Murgič, Chem. Biochem. Eng. 27, 65, (2013).
- D. Carta, G. Cao, C. D`Angeli, Environ. Sci. Pollut. R. 10, 390, (2003).
- Ch. H. Chen, J. Appl. Polym. Sci. 87, 2004, (2003).
- B. Geyer, G. Lorenz, A. Kandelbauer, Express Polym. Lett. 10, 559, (2016).
- M.E. Viana, A. Riul, G.M. Carvalho, A.F. Rubira, E.C. Muniz, Chem. Eng. J. 173, 210, (2011).
- N. George, T. Kurian, Ind. Eng. Chem. Res. 53, 14185, (2014).
- N.D. Pingale, V.. Palekar, S.R. Shukla, J. Appl. Polym. Sci. 115, 249, (2010).
- M. Ghaemy, F. Behzadi, Iran. Polym. J. 11, 77, (2002).
- R. López-Fonseca, I. Duque-Ingunza, B. Rivas, S. Arnaiz, J. Gutierrez- Ortiz, Polym. Degrad. Stab. 95, 1022, (2010).
- M.E. Viana, A. Riul, G.M. Carvalho, A.F. Rubira, E.C. Muñiz, Chem. Eng. J. 173, 210, (2011).
- M.Y. Abdelaal, T.R. Sobahi, M.S. Makki, Constr. Build. Mater. 25, 3267, (2011).
- S.R. Shukla, A.M. Harad, J. Appl. Polym, Sci. 97, 513, (2005).
- H. Wang, Y. Liu, Z. Li, X. Zhang, S. Zhang, Y. Zhang, Eur. Polym. J. 45, 1535, (2009).
- M. Zhu, S. Li, Z. Li, X. Lu, S. Zhang, Chem. Eng. J. 185, 168, (2012).
- K. Fukushima, O. Coulembier, J.M. Lecuyer, H.A. Almegren, A.M. Alabdulrahman, F.D. Alsewailem, M.A. Mcneil, P. Dubois, R.M. Waymouth, H.W. Horn, J.E. Rice, J.L. Hedrick, J. Polym. Sci. Part A: Polym. Chem. 49, 1273, (2011).
- V. Sharma, P. Shrivastava, D.D. Agarwal, J. Polym. Res. 22, 241, (2015).
- M.A. Alnaqbi, M.A. Mohsin, R.M. Busheer, Y. Haik, J. Appl. Polym. Sci. Doi: 10.1002/APP.41666, (2015).
- M.Y. Abdelaal, T.R. Sobahi, M.S.I. Makki, Constr. Build. Mater. 25, 3267, (2011).
- A.R. Zahedi, M. Rafizadeh, F.A. Taromi, J. Thermoplast. Compos. Mat. 27,1256, (2014).
- P. Elizondo, B. Nájera, N.A. Pérez, L. Hinojosa, M.I. Gómez J. Serb. Chem. Soc. 78, 591, (2013).
- V. Pimpa, R. Sirisook, S. Chuayjuljit, J. Appl. Polym. Sci. 88, 788, (2003).
- R.M.K. Prado, Ch.R. Nascimento, Ch. Azuma, M.L. Dias, Prog. Rubber. Plast. Re. 24, 183, (2008).


