ELECTROOXIDATION OF SULFITE AT CARBON PASTE ELECTRODE MODIFIED WITH IONIC LIQUIDS DERIVATED OF N-OCTYL-PYRIDINIUM HEXAFLUOROPHOSPHATE WITH DIFFERENT SUBSTITUENTS IN THE CATION
- Ionic liquids,
- N-octyl-pyridinium hexafluorophospate,
- Composite ionic-liquid electrodes CILEs,
- Carbon paste electrode CPE,
- Sulfite oxidation
- Effect of the substituent ...More
Copyright (c) 2018 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
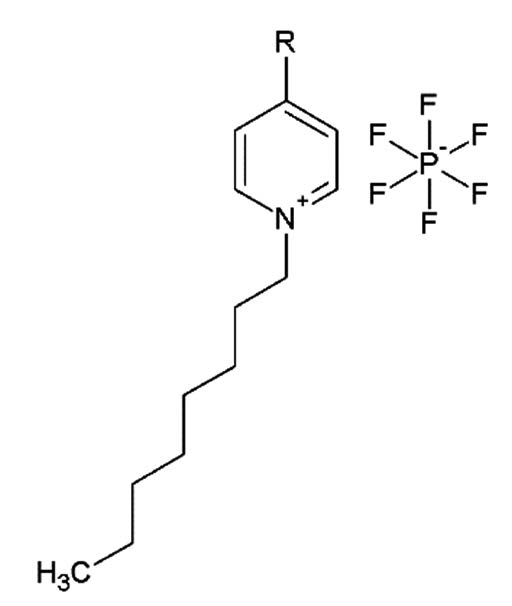
Carbon paste electrodes modified with a family of ionic liquids (as binders) derived from N-octyl-pyridinium hexafluorophosphate, (composite-ionic liquid electrodes (CILEs)) were studied toward the oxidation of sulfite as an inner-sphere probe reaction and compared to a conventional carbon paste electrode (CPE) in which the binder was mineral oil. The ionic liquids were modified at para-position with substituents that are electron withdrawing: -CN (CILE/CN) and –CF3 (CILE/CF3) and substituents that are electron donating: -CH3 (CILE/CH3) and –OCH3 (CILE/OCH3), and compared with the ionic liquid without substituents (CILE/OPy). The results showed that CILEs are capable of catalyzing the sulfite oxidation, shifting the oxidation potential to more negative values compared to CPE. Also, they showed linear correlations between increasing sulfite concentration and increasing current density. The best system in terms of sensitivity was the electrode modified with the 4-methyl-N-octylpyridiniumhexafluorophosphate CILE/CH3 and, then, that modified with the non-substituted ionic liquid CILE/OPy measured by amperometry. In terms of potential, the best systems are the CILEs modified with ILs with inductive-substituents, -CH3 and –CF3 and OPy, indicating that the delocalization of the charge of the cation produced by the mesomeric-substituent (-OCH3, -CN) diminishes the electrocatalytic behavior of these binders for that oxidation. On the other hand, the analytical parameters of all the amperometric sensors studied here (all CILEs excepts CILE/OCH3) are good enough to be applied in food industry for samples without polyphenols at concentrations higher than ca. 1mM of sulfite. Their stability is very high (at least 100 cycles after obtaining a stable response without changing its current, exposed to air and humidity) and they can be used to remove sulfite from wastewaters. Finally, to our knowledge, there are not comparative studies about the effect of changing the substituent of the cation in electrocatalysis of modified IL-electrodes.
References
- F. Faridbod, M. Reza, P. Norouzi, S. Riahi, H. Rashedi in Application of room temperature ionic liquids in electrochemical sensors and biosensors. Ionic Liquids: Applications and Perspectives. 2011, pp.643-658.
- A. Mehrkesh, A. Karunanithi, Fluid Phase Equilib. 427,498 (2016).
- T.Tsuda, C. Hussey in Electrochemical Applications of Room-Temperature Ionic Liquids. Electrochem. Soc. Interface. 2007 pp. 42-49.
- A. Franco-Vega, E. Palou, N. Ramirez-Corona, A. Lopez-Malo in Líquidos iónicos: una alternativa verde para procesos de extracción en la industria de alimentos. Temas selectos de ingeniería de alimentos. 2014, vol. 1, pp.15-26.
- S. Vishwakarma IJBSAC.1, 1, (2014).
- P. Hapiot, C. Lagrost, Chem. Rev. 108, 2238, (2008).
- X. Cao, X. He, J. Wang, H. Liu, S. Röser, B. Razaei Rad, M. Evertz, B. Streipert, J. Li, R. Wagner, M. Winter, I. Cekic-Laskovic. ACS App. Mater. Interfaces. 8, 25971 (2016).
- M.Opallo, A. Lesniewski, J. Electroanal. Chem. 656, 2, (2011).
- M.Shiddiky, A Torriero, Biosens. Bioelectron. 26,1775, (2011).
- N. Maleki, A. Safavi, ; F. Tajabadi, Anal. Chem. 78, 3820, (2006).
- M. Shamsipur, A. Pashabadi, A. Taherpour, B. Hemmateenejad, T. Khosousi, M. Hadi Parvin, J. Electroanal. Chem. 778, 116 (2016).
- M. Godindhan, A. Chen, Microchim. Acta. 183, 2879 (2016).
- A. Safavi, N. Maleki, F. Honarasa, F. Tajabadi, F. Sedaghatpour, Electroanal.19, 582, (2007).
- A. Safavi , N. Maleki, O. Moradlou, F. Tajabadi, Anal. Biochem. 359, 224, (2006).
- E. Bordeu and J. Scarpa. Análisis Químico del vino. Ediciones Universidad Católica de Chile, Santiago 2000.
- F. Zamora in Anhídrido sulfuroso; Algunas reflexiones sobre este aditivo. Revista enólogos. 2005, no. 38. pp. 28-31.
- R. Jackson in Wine science principles and applications. Elsevier, San Diego, 2014.
- Y.Yang, Y. Yan, X. Chen, W. Zhai, Y. Xu, Y. Liu, Electrocatal. 5, 344 (2014).
- E. Silva, R. Takeuchi, A. Santos. Food Chem. 173, 763 (2015).
- B. Devadas, M. Sivakumar, S. Ming Chen, S. Cheemalapati. Electrochim. Acta, 176, 350 (2015).
- L. Xu, F. Guo, Y. You, J. Hu, Y. Miao, Z. Wu, L. Wang. Int. J. Electrochem. Sci. 11, 4586 (2016).
- W. Syrosee, K. Ponlakhet, S. Charaim, P. Jarujamrus, M. Amatatongchai, Talanta, 156-157,154 (2016).
- D. Fang, J. Cheng, K. Gong, Q. Shi, L. Zhou, Z. Liu, J. Fluorine Chem. 129, 108, (2008).
- U. Domańska, K. Skiba, M. Zawadzki, K. Paduszyńsky, M. Królikowski. J. Chem. Thermodyn. 56, 53, (2013).
- R. Arce, MJ. Aguirre, J. Romero. Int. J. Electrochem. Sci. 9, 7916 (2014).
- R. Arce, J. Romero, M J. Aguirre. J. Appl. Electrochem. 44, 1361 (2014).
- R. Arce, C. Baéz, J. P. Muena, M J. Aguirre, J. Romero. J. Chil. Chem. Soc. 61, 3014, (2016).
- J.C. Miller, J.N. Miller in Estadística para química analítica. EUA Eds. Addison Wesley, Iberoamericana, 1988.
- Ley 18.455, Decreto 78, Normas sobre producción, elaboración y comercialización de alcoholes etílicos, bebidas alcohólicas y vinagres.


