SYNTHESIS AND ANTIMICROBIAL EVALUATION OF SOME NEW ASYMMETRICALLY SUBSTITUTED 4-ARYL- 2,6-DI(COUMARINYL) PYRIDINES
- Dicoumarinyl pyridines,
- Krohnke’s reaction,
- Antimicrobial activity,
- Broth dilution method
Copyright (c) 2016 Yogita L. Chovatiya, Kaushik N. Kundaliya, Rakesh R. Giri, Dinker I. Brahmbhatt

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Abstract
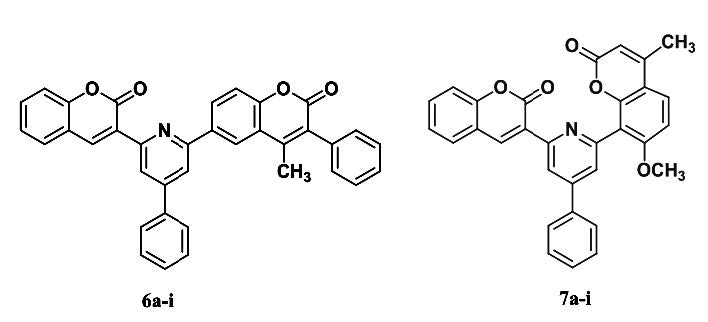
In the present work the synthesis of various 4-aryl-2-(coumarin-3-yl)-6-(4-methyl-3-phenyl coumarin-6-yl)pyridines (6a-i) and 4-aryl-2-(coumarin-3-yl)- 6-(4-methyl-7-methoxy coumarin-8-yl)pyridines (7a-i) have been carried out by reacting 1-[2(H)-1-benzopyran-3-yl]-3-aryl-prop-2-en-1-ones (coumarinoyl chalcones) (3a-f) with appropriate coumarinoyl methyl pyridinium bromide salt 4 and 5 respectively. The target compounds were characterized by the IR, 1H-NMR, 13C-APT and mass spectral analysis. Preliminary examination of target compounds as antimicrobial agents has been carried out using Broth dilution method.
References
- -S. Sandhu, Y. Bansal, O. Silakari, G. Bansal, Bioorg. Med. Chem. 22, 3806, (2014).
- -S. Rosselli, A. Maggio, G. Bellone, C. Formisano, A. Basile, C. Cicala, A. Alfieri, N. Mascolo, M. Bruno, Planta. Med. 73, 116, (2007).
- -A. Kamal, S. F. Adil, J. R. Tamboli, B. Siddardha, U. S. N. Murthy, Lett. Drug. Des. Discov. 6, 201, (2009).
- -K. V. Sashidhara, M. Kumar, R. K. Modukuri, R. Sonkar, G. Bhatia, A. K. Khanna, S. Rai, R. Shukla, Bioorg. Med. Chem. Lett. 21, 4480, (2011).
- -K. V. Shashidhara, A. Kumar, M. Kumar, A. Srivastva, A. Puri, Bioorg. Med. Chem. Lett. 20, 6504, (2010).
- -M. Campos-Toimil, F. Orallo, L. Santana, E. Uriarte, Bioorg. Med. Chem. 12, 783, (2002).
- -R. B. Moffett, J. Med. Chem. 7, 446, (1964).
- -H. Osman, A. Arshad, C. K. Lam, M. C. Bagley, Chem. Cent. J. 6, 32, (2012).
- -A. Manvar, A. Malde, J. Verma, V. Virsodia, A. Mishra, K. Upadhyay, H. Acharya, E. Coutinho, A. Shah, Eur. J. Med. Chem. 43, 2395, (2008).
- -R. Argotte-Ramos, G. Ramírez-Avila, M. Rodríguez-Gutiérrez , M. Ovilla- Muñoz, H. Lanz-Mendoza , M. H. Rodríguez , M. González-Cortazar, L. Alvarez. J. Nat. Prod. 69, 1442, (2006).
- -R. B. Moffett, U. S. Patent, 3, 156, 697, (1964).
- -B. Sreenivasulu, V. Sundaramurthy, R. N. V. Subba, Proc. Ind. Acad. Sci., Sec. A. 79, 41, (1974).
- -R. B. Moffett, U. S. Patent, 3, 201, 406, (1965).
- -R. B. Moffett, J. Med. Chem. 7, 446, (1964).
- -D. I. Brahmbhatt, C. V. Patel, V. G. Bhila, N. H. Patel, A. A. Patel, Med. Chem. Res. 24 1596, (2015).
- -H. B. Lad, R. R. Giri, D. I. Brahmbhatt, Chin. Chem. Lett. 24, 227, (2013).
- -V. G. Bhila, C. V. Patel, N. H. Patel, D. I. Brahmbhatt, Med. Chem. Res. 22, 4338, (2013).
- -N. H. Patel, A. K. Patel, C. V. Patel, A. A. Patel, D. I. Brahmbhatt, Arkivoc ii, 283, (2010).
- -D. I. Brahmbhatt, J. M. Gajera, V. P. Pandya, M. P. Patel, Ind. J. Chem. 46B, 869, (2007).
- -D. I. Brahmbhatt, V. P. Pandya, C. N. Patel, M. A. Patel, Ind. J. Chem. 44B, 1863, (2005).
- -R. R. Giri, H. B. Lad, V. G. Bhila, C. V. Patel, D. I. Brahmbhatt, Synth. Commun. 45(3), 363, (2015).
- -H. B. Lad, R. R. Giri, Y. L. Chovatiya, D. I. Brahmbhatt, J. Serb. Chem. Soc. doi: 10.2298/JSC140804004L.
- -A. K. Patel, N. H. Patel, M. A. Patel, D. I. Brahmbhatt, Arkivoc xi, 28, (2010).
- -F. Krohnke, Synthesis, 1, 1, (1979).
- -NCCLS (National Committee for Clinical Laboratory Standards) (2002) 940, West Valley Road, Suite 1400, Wayne, Pennsylvania 19087–1898, USA. Performance Standards for Antimicrobial Susceptibility Testing; Twelfth Informational Supplement (ISBN 1-56238-454-6), M100–S12 (M7).
- -C. F. Koelsch, J. Am. Chem. Soc. 72, 2993, (1950).
- -D. I. Brahmbhatt, B. R. Hirani, Ind. J. Chem., Sect. B. 33, 1072, (1994).


