ABSOLUTE CONFIGURATION ASSIGNMENT IN RACEMIC trans-STILBENE OXIDE USING CHIRAL LIQUID CHROMATOGRAPHY WITH COMBINED CHIROPTICAL DETECTION AND TIME-DEPENDENT DENSITY FUNCTIONAL THEORY CALCULATIONS
Copyright (c) 2016 Juan P. Castillo-González, Natalia I. González-Peña, Marcelo A. Muñoz

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Abstract
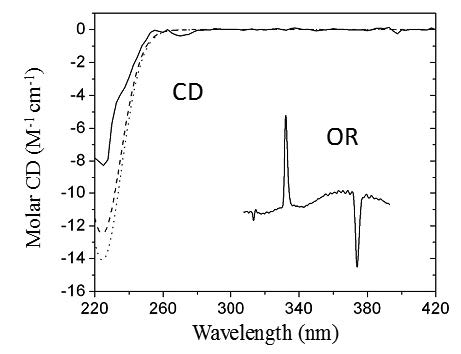
A chiral chromatographic analysis using HPLC coupled in series with circular dichroism and optical rotation detectors (HPLC-CD-OR) was applied to racemic trans-stilbene oxide, allowing the on-line measurement of the CD spectra and [α]670 values for both enantiomers in the sample. These measurements used the Gaussian peak model to estimate the concentration of each isomer in the CD and OR cells. Moreover, time-dependent density functional theory (TD-DFT) calculations at the B3LYP/TZVP//B3LYP/VDZ(P) and B3LYP/aug-cc-pVDZ//B3LYP/VDZ(P) levels of theory for CD and OR, respectively, permitted the prediction of both chiroptical properties for each isomer, which in turn could be compared with the corresponding experimental data. In this case, the calculations were consistent in predicting the correct combination of experimental CD bands and [α]670 sign and therefore facilitated the absolute configuration (AC) assignment of the optical isomers. The AC proposed using this methodology was in agreement with independent AC assignments in the literature.
References
- -Q. Shen, L. Wang, H. Zhou, H. Jiang, L. Yu, Su Zeng, Acta Pharmacol. Sin. 34, 998-1006, (2013).
- -H.D. Flack, G. Bernardinelli, Chirality 20, 681-690, (2008).
- -G.H. Wagnière, On the interaction of light with molecules: pathways to the theoretical interpretation of chiroptical phenomena, in: N. Berova, P.L. Polavarapu, K. Nakanishi, R.W. Woody (Eds.), Comprehensive Chiroptical Spectroscopy Vol. 1, John Wiley & Sons, New York, 2012, pp. 3-34.
- -L. Kott, W.B. Holzheuer, M.M. Wong, G.K. Webster, J. Pharm. Biomed. Anal. 43, 57-65, (2007).
- -X.-M. Chena, C. Yamamotob, Y. Okamoto, J. Chromatogr. A 1104, 62-68, (2006).
- -E. Bossu’, V. Cotichini, G. Gostoli, A. Farina, J. Pharm. Biomed. Anal. 26, 837-848, (2001).
- -G. Bringmann, T.A.M. Gulder, M. Reichert, T. Gulder, Chirality 20, 628- 642, (2008).
- -N. Vanthuyne, C. Roussel, Chiroptical detectors for the study of unusual phenomena in chiral chromatography, in: V. Schurig (Ed.), Differentiation of Enantiomers I, Topics in Current Chemistry Volume 340, Springer International Publishing, 2013, pp. 107-151.
- -K. Szwed, M. Górecki, J. Frelek, M. Asztemborska, Chromatographia 76, 1603-1611, (2013).
- -P.J. Stephens, J.J. Pan, F.J. Devlin, K. Krohn, T. Kurta´n, J. Org. Chem. 72, 3521-3526, (2007).
- -P.J. Stephens, J.-J. Pan, F.J. Devlin, M. Urbanová, J. Hájíček, J. Org. Chem. 72, 2508-2524, (2007).
- -P.J. Stephens, F.J. Devlin, F. Gasparrini, A. Ciogli, D. Spinelli, B. Cosimelli, J. Org. Chem. 72, 4707-4715, (2007).
- -P.J. Stephens, D.M. McCann, F.J. Devlin, A.B. Smith III, J. Nat. Prod. 69, 1055-1064, (2006).
- -M.A. Muñoz, M.A. Bucio, P. Joseph-Nathan, Nat. Prod. Commun. 8, 1075-1078, (2013).
- -C. Bertucci, D. Tedesco, J. Chromatogr. A 1269, 69-81, (2012).
- -P.D. Rice, Y.Y. Shao, S.R. Erskine, T.G. Teague, D.R. Bobbitt, Talanta 36, 473-478, (1989).
- -S. Sinnecker, A. Rajendran, A. Klamt, M. Diedenhofen, F. Neese, J. Phys. Chem. A. 110, 2235-2245, (2006).
- -T. Bruhn, A. Schaumlöffel, Y. Hemberger, G. Bringmann, SpecDis version 1.62, University of Wuerzburg, Germany, 2014. (http://www-organik. chemie.uni-wuerzburg.de/lehrstuehlearbeitskreise/bringmann/specdis/).
- -R. Bauernschmitt, R. Ahlrichs, Chem. Phys. Lett. 256, 454-464, (1996).
- -M. Srebro, N. Govind, W. A. de Jong, J. Autschbach, J. Phys. Chem. A 115, 10930-10949, (2011).
- -F. Neese, WIREs Comput. Mol. Sci. 2, 73-78, (2012).
- -M. Valiev, E.J. Bylaska, N. Govind, K. Kowalski, T.P. Straatsma, H.J.J. van Dam, D. Wang, J. Nieplocha, E. Apra, T.L. Windus, W.A. de Jong, Comput. Phys. Commun. 181, 1477-1489, (2010).
- -N. Berova, L.D. Bari, G. Pescitelli, Chem. Soc. Rev. 36, 914-931, (2007).
- -A.L. Slitt, N.J. Cherrington, M.Z. Dieter, L.M. Aleksunes, G.L. Scheffer, W. Huang, D.D. Moore, C.D. Klaassen, Mol. Pharmacol. 69, 1554-1563, (2006).
- -Lux Columns Complete Chiral Solutions Catalogue, Phenomenex, Torrance, CA, USA (2013).
- -G. Gottarelli, S. F. Mason, G. Torre, J. Chem. Soc. B, 1349-1353, (1970).
- -K. Mislow, Introduction to Stereochemistry, first ed., W. A. Benjamin, Inc., New York, 1966.
- -M. Imuta, H. Ziffer, J. Org. Chem. 44, 2505-2509, (1979).


