LIQUID CHROMATOGRAPHIC DETERMINATION OF SOLIFENACIN SUCCINATE, FLAVOXATE HYDROCHLORIDE AND TOLTERODINE TARTRATE IN BULK DRUGS AND THEIR PHARMACEUTICAL DOSAGE FORMS
- Solifenacin succinate,
- Flavoxate HCl,
- Toltoridine tartarate,
- RP-HPLC
Copyright (c) 2016 Ali K. Attia, Eman Y. Z. Frag, Gehad G. Mohamed, Heba E. Ahmed

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Abstract
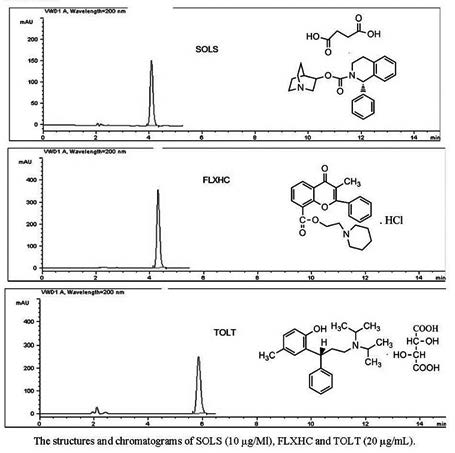
A simple, precise, specific and accurate reversed phase HPLC (RP-HPLC) method has been developed for the determination of solifenacin succinate (SOLS), flavoxate HCl (FLXHC) and toltoridine tartarate (TOLT) in bulk and pharmaceutical dosage forms. The proposed RP-HPLC method was carried out using Xterra RP-18 column (5 μm practical size, 25 cm x 4.6 mm i.d.). The flow rate, the injection volume and the detection wavelength were 1.0 mL/min, 20 mL and 200 nm, respectively. The mobile phase consisted of 0.05 M pentane sulfonic acid sodium salt (SOLS: pH 3.0±0.05, FLXHC and TOLT: pH 5.5±0.05) and acetonitrile (50:50 v/v). The retention times for SOLS, FLXHC and TOLT drugs were found to be 4.1±0.1 min, 4.3±0.0 min and 5.8±0.1 min, respectively. The calibration was linear over the concentration range of 0.1-100 μg/mL. The mean recoveries for SOLS, FLXHC and TOLT drugs were about 99.80%, 100.43% and 100.00%, respectively. The method was validated according to the ICH guidelines with respect to specificity, linearity, accuracy, precision and robustness.
References
- -M. Maniscalco, D. S. Franco, W. R. Wolowich, R. T. Colon, Clin. Ther. 28, 1247, (2006).
- -K. J. Kreder, Urol. Clin. North Am. 33, 483, (2006).
- -S. C. Sweetman, Martindale, The Complete Drug Reference, 36th ed. Pharmaceutical Press, London, 2009.
- -B. K. Malhotra, P. Glue, K. Sweeney, R. Anziano, J. Mancuso, P. Wicker, Clin. Pharmacol. Therap. 81, 377, (2007).
- -M. J. O. Neil, The Merck index, An Encyclopedia of Chemicals, Drugs and Biological. 14th ed. New Jersey: Merck Research Laboratories, Whitehouse station, 2006.
- -B. V. R. Reddy, B. S. Reddy, N. V. V. S. S. Raman, R. K. Subhash, C. Rambabu, J. Chem. 2013, 1, (2013).
- -M. M. Annapurna, G. Sowjanya, M. S. Naidu, D. Lohithasu, Chem. Sci. Trans. 3, 602, (2014).
- -V. Vijayasree, D. A. Kumar, J. V. L. N. S. Rao, Pharmanest 4, 206, (2013).
- -S. R. Krishna, B. M. Rao, N. S. Rao, J. Chromatogr. Sci. 48, 807, (2010).
- -N. Desai, S. H. Syed, S. G. Vasanthraju, A. Karthik, N. Udupa, Int. J. Pharm. Pharm. Sci. 3, 70 (2011).
- -D. S. Israel, K. Krishnachaitanya, D. Gowrisankar, IJPSR, 4, 4343, (2013).
- -N. Paliwal, P. Jain, N. Dubey, S. Sharma, S. Khurana, R. Mishra, S.K. Paliwal, Int. J. Drug Dev. Res. 5, 91, (2013).
- -P. S. Babu, SK. R. Parveen, K. B. Chandrasekar, B. R. Challa, B. Z. Sh. Awen, JSRR, 3, 1905, (2014).
- -S. B. Puttagunta, R. P. Shaik, C. K. Bannoth, B. S. R. Challa, B. Z. Sh. Awen, J. Anal. Sci. Tech. 5, 1, (2014).
- -T. Oki, S. Sato, K. Miyata, S. Yamada, British J. Pharmacol. 145, 219, (2005).
- -D. Desai, G. Mehta, D. Ruikar, R. A Jain, S. J. Rajput, Asian J. Pharm. Biol. Res. 1, 310, (2011).
- -S. B. Wankhede, K. Somani, S. S. Chitlange, Int. J. Chem. Tech. Res. 3, 2003, (2011).
- -M. V. Attimarad, N. S. Harsha, R. S. Setty, J. Liq. Chromatogr. Rel. Tech. 35, 768, (2012).
- -A. El-Gindy, R. A. Abdel-Salam, S. Sallam, Drug Dev. Ind. Pharm. 34, 1311, (2008).
- -A. Mahesh, J. Young Pharm. 2, 280, (2010).
- -R. N. El-Shaheny, N. M. El-Enany, F. F. Belal, Anal. Methods 6, 1001, (2014).
- -S. A. Kumar, M. Debnath, J. V. L. N. S. Rao, Int. J. Pharm. Pharm. Sci. 5, 665, (2013).
- -S. K. R. Parveen, P. S. Babu, K. B. Chandrasekar, B. R. Challa, Der Pharmacia Lett. 6, 246, (2014).
- -N. Ramathilagam, M. Meeradevi, P. Solairaj, S. C. Rajesh, Int. J. Pharm. Bio. Sci. 2, 332, (2012).
- -S. K. Shetty, A. Shah, Int. J. Pharm. Tech. Res. 3, 1083, (2011).
- -S. R. Krishna, B. M. Rao, N. S. Rao, Rasayan J. Chem. 2, 144, (2009).
- -S. M. Mhamunkar, R. Y. Vyavaharkar, S. Bhoir, Int. J. Pharm. Pharm. Sci. 4, 319, (2012).
- -M. V. B. Rao, B. C. Reddy, T. S. Rao, J. R. Mohanty, Res. J. Pharm. Bio. Chem. Sci. 1, 136, (2010).
- -R. Yanamandra, C. S. Vadla, U. Puppala, B. Patro, Y. L. N. Murthy, P. A. Ramaiah, Sci. Pharm. 80, 101, (2012).
- -G. D. Teja, Ch. D. Dasu, P. S. Babu, P. Ravisankar, JCPS, 6, 195, (2013).
- -R. Seetharaman, K. S. Lakshmi, Int. J. Res. Pharm. Biomed. Sci. 2, 1052, (2011).
- -M. Attimarad, J. Basic Clin. Pharm. 2, 53, (2011).
- -M. Attimarad, J. Iranian Chem. Soc. 9, 551, (2012).
- -S. K. Shetty, A. Shah, IJPSR, 2, 1456, (2011).
- -S. Vanilatha, M. M. Theresa, N. Prasanna, D. S. Kumari, B. Harika, P. Sirisha, M. A. Xavier, K. G. B. Kumari, IJSID, 1, 288, (2011).
- -S. M. Fraihat, H. S. Khatib, Asian J. Chem. 25, 1887, (2013).
- -M. S. Rizk, F. M. Abdel-Haleem, Electrochim. Acta 55, 5592, (2010).
- -M. M. Sakr, R. M. El Nashar, Talanta 96, 153, (2012).
- -M. W. Nassar, K. A. Attia, H. M. Abou-Seada, A. E. Allam, IJPSR, 4, 3845, (2013).
- -M. M. Ghoneim, M. A. El-Attar, S. A. Razeq, Central Eur. J. Chem. 5, 496, (2007).
- -D. Kul, J. Anal. Chem. 69, 970, (2014).
- -P. Macikova, J. Skopalova, P. Cankar, B. Papouskova, R. Strakova, D. Jirovsky, V. Maier, Electroanalysis 25, 205, (2013).
- -ICH, Stability Testing of New Drug Substances and Products. International Conference on Harmonization, IFPMA, Geneva, 1993.


