ARSENIC SORPTION USING MIXTURES OF ION EXCHANGE RESINS CONTAINING N-METHYL-DGLUCAMINE AND QUATERNARY AMMONIUM GROUPS
- Arsenic,
- N-methyl-D-glucamine,
- Ion exchange,
- Ion exchange resin mixture
Copyright (c) 2016 Gulsah Ozkula, Bruno F. Urbana, Bernabé L. Rivas, Nalan Kabay, Marek Bryjak

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Abstract
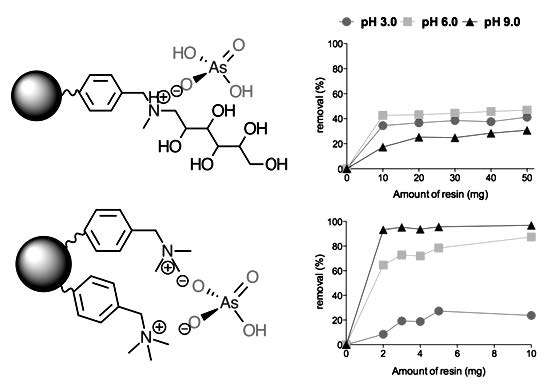
The method of synthesis and the arsenic removal properties of ion-exchange resins based on N-methyl-D-glucamine and trimethylammonium groups are presented. The N-methyl-D-glucamine based monomer was synthesized by the reaction of 4-vinyl benzyl chloride with N-methyl-D-glucamine, along with the use of N,N-methylene-bis-acrylamide as a crosslinker reagent for polymerization. In addition, poly(4-vinylbenzyl)trimethylammonium was synthesized. Arsenate sorption studies were conducted and the pH effect, kinetics, sorption capacity, and elution performance were studied. The experimental data were fitted to kinetic models, such as the pseudo-first order and pseudo-second order models. The pseudo-second order model exhibited the best correlation with the experimental data. The Langmuir and Freundlich isotherms were fitted to the experimental data, and the Freundlich isotherm exhibited the best fit.
References
- -C. S. Brooks, Metal recovery from industrial waste, Lewis Publishers (1991).
- -R. A. Beauvais and S. D. Alexandratos, React Funct Polym 36:113-123 (1998).
- -D. C. Sharma and C. F. Forster, Water Research 27:1201-1208 (1993).
- -P. Udaybhaskar, L. Iyengar and A. V. S. P. Rao, J Appl Polym Sci 39:739- 747 (1990).
- -E. Guibal, C. Milot and J. M. Tobin, Ind Eng Chem Res 37:1454-1463 (1998).
- -Y. Marcus and A. S. Kertes, Ion exchange and solvent extraction of metal complexes, Wiley-Interscience (1969).
- -K. E. Geckeler, R. Zhou and B. L. Rivas, Die Angew. Makromol Chem. 197:107-115 (1992).
- -B. L. Rivas, H. A. Maturana and E. Pereira, Die Angew Makromol Chem. 220:61-74 (1994).
- -B. L. Rivas and I. Moreno-Villoslada, Journal of J. Membrane Sci. 178:165-170 (2000).
- -B. L. Rivas, H. A. Maturana, R. E. Catalán and I. M. Perich, J Appl Polym Sci 38:801-807 (1989).
- -B. L. Rivas and G. V. Seguel, Polyhedron 18:2511-2518 (1999).
- -B. L. Rivas and I. Moreno-Villoslada, Chem Lett 166-167 (2000).
- -B. L. Rivas and C. O. Sánchez, J Appl Polym Sci 89:2641-2648 (2003).
- -I. Moreno-Villoslada and B. L. Rivas, The J. of Phys. Chem. B 106:9708- 9711 (2002).
- -K. Henke, Arsenic, John Wiley & Sons Ltd, Chichester, West Sussex, UK. (2009).
- -H. V. Aposhian and M. M. Aposhian, Chem Res Toxicol 19:1-15 (2006).
- -M. Bissen and F. H. Frimmel, Acta Hydrochim Hydrobiol 31:9-18 (2003).
- -B. K. Mandal and K. T. Suzuki, Talanta 58:201 - 235 (2002).
- -U. EPA, Proven Alternatives for Aboveground Treatment of Arsenic in Groundwater. US EPA (2002).
- -S. Amrose, A. Gadgil, V. Srinivasan, K. Kowolik, M. Muller, J. Huang and R. Kostecki, J Environ Sci Health, Part A 48:1019-1030 (2013).
- -M. Bissen and F. H. Frimmel, Acta Hydrochim Hydrobiol 31:97-107 (2003).
- -S. D. Alexandratos, J Hazard Mater A139:467 - 470 (2007).
- -F. F. Chang and W. J. Liu, Water Sci Technol 65:296-302 (2012).
- -L. Dambies, Sep Sci Technol 39:603 - 627 (2004).
- -U. E. P. AgencyEPA-542-S-02-002:Solid Waste and Emergency Response (5102G) (2002).
- -E. P. Agency, Arsenic Treatment Technology Evaluation Handbook for Small Systems. EPA U.S. (2003).
- -D. A. Clifford and G. L. Ghurye, Metal-Oxide Adsorption, Ion Exchange, and Coagulation–Microfiltration for Arsenic Removal from Water, in Environmental Chemistry of Arsenic, ed by W. T. Frankenberger. Marcel Dekker, Inc. , New York, pp. 217 (2002).
- -L. Dambies, R. Salinaro and S. Alexandratos, Environ Sci Technol 38:6139 - 6146 (2004).
- -B. F. Urbano, B. L. Rivas, F. Martinez and S. D. Alexandratos, React Funct Polym 72:642-649 (2012).
- -B. Urbano, B. L. Rivas, F. Martinez and S. D. Alexandratos, Chem Eng J 193 - 194:21 - 30 (2012).
- -L. Toledo, B. L. Rivas, B. F. Urbano and J. Sánchez, Sep Purif Technol 103:1-7 (2013).
- -Y. S. Ho, J Hazard Mater B136:681 - 689 (2006).
- -Y. S. Ho, Scientometrics 59:171-177 (2004).
- -F. Helfferich, Ion Exchange, Dover Publication Inc., New York (1962).
- -Y. S. Ho, J. C. Y. Ng and G. McKay, Sep Purif Methods 29:189 - 232 (2000).
- - Y. S. Ho and G. McKay, Process Biochem 34:451 - 465 (1999).
- -A. A. Zagorodni, Ion Exchange Materials Properties and Applications, Elsevier BV, Amsterdam (2007).
- -E. Korngold, N. Belayev and L. Aronov, Desalination 141:81-84 (2001).
- -A. Chiavola, E. D’Amato and R. Baciocchi, Water, Air, Soil Pollut 223:2373-2386 (2012).
- -M. R. Awual, M. A. Hossain, M. A. Shenashen, T. Yaita, S. Suzuki and A. Jyo, Environ Sci Pollut Res 20:421-430 (2013).
- -A. M. Donia, A. A. Atia and D. H. Mabrouk, J Hazard Mater 191:1-7 (2011).


