- absolute configuration,
- diterpenoid,
- X-ray diffraction,
- crystal and supramolecular structure
Copyright (c) 2017 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
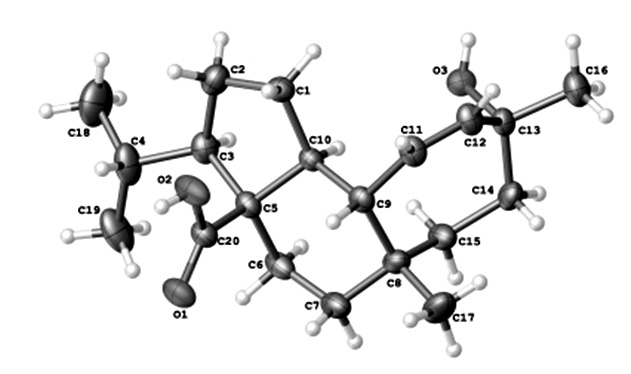
The structure of this mulinolic acid consists of a mulinane skeleton and the corresponding isopropyl, methyl, carboxyl and methyl groups at C3, C8, C5, C13, respectively, which are β-oriented, whereas the hydroxyl group at C13 is α-oriented. The cyclopentane (A), ciclohexane (B) and cicloheptene (C) rings are trans (A/B) and (B/C) cis fused, and in an envelope, chair, and twist conformation respectively.
In the crystal the molecules are linked by two intermolecular O-H⋅⋅⋅O hydrogen bond forming bidimensional supramolecular structures with graph-set notation R44(12), R44(40), R66(46) and C44(46). The absolute configuration of the title compound has been determined from the refinement of the Flack parameter16. On this basis the absolute configuration was assigned as C3R, C5S, C8S, C9S, C10S and C13R.
References
- C. Munizaga, H. Gunkel, Notas etnobotánicas del pueblo atacameño de Socaire. Publicación No. 5. Universidad de Chile, 1958.
- J.Bórquez, A. Ardiles, L. A. Loyola, L.M.Peña-Rodriguez, G.M. Molina- Salinas, J. Vallejos, I.G.Collado, M.J. Simirgiotis. Molecules, 19, 3898, (2014).
- I.Brito, M. J. Simirgiotis, A. Brito, Werner, M. R., Bórquez, J., Winterhalter, P., A. Cárdenas. Journal of the Chilean Chemical Society 60, (1), 2864, (2015).
- I.Brito, J. Bórquez, M.Simirgiotis, M. Neves-Vieira, G.Jerz, P.Winterhalter, M. Bolte, A. Cárdenas. Zeitschrift für Kristallographie- New Crystal Structures 229, 399, (2014).
- J.,Bórquez, N.L. Bartolucci, C. Echiburú-Chau, P.Winterhalter, J. Vallejos, G. Jerz, M.J. Simirgiotis. Journal of the Science of Food and Agriculture 96, (8), 2832, (2016).
- L. A. Loyola, J. Bórquez, G. Morales, A. San Martín. Phytochemistry, 44, 649, (1997).
- L. A. Loyola, J. Bórquez, G. Morales, A. San Martín. Phytochemistry, 4, (1), 165, (1996).
- L.A. Loyola, J.Bórquez, G. Morales, A. San Martín., Phytochemistry, 53, 96, (2000).
- L.A. Loyola, J. Bórquez, G.Morales, A. San Martín. Phytochemistry, 45 (7), 1465, (1997).
- I. Brito, J. Bórquez, L. A. Loyola, A. Cárdenas, M. López-Rodríguez. Acta cryst, E 64, o1209, (2008).
- I. Brito, J. Bórquez, J.Albanez, M. Bolte, L. M. Peña-Rodriguez. Acta Cryst, E 66, 02452, (2010).
- G. M. Sheldrick, Acta Cryst. 112 , A64, (2008).
- O.V. Dolomanov, L.J. Bourhis, R.J. Gildea, J.A.K. Howard, H. Puschmann, J. Appl. Cryst., 42, 339, (2009).
- D.Cremer, J.A. Pople. J.Am.Chem.Soc. 97, 1354, (1975).
- H.D. Flack. Acta Cryst. A39, 876, (1983).
- J. Bernstein, R.E. Davis, L. Shimoni, N.-L. Chang. Angew. Chem. Int. Ed. Engl. 34, 1555, (1995).


