EFFICIENT REMOVAL OF Cr(VI) BY POLYELECTROLYTE-ASSISTED ULTRAFILTRATION AND SUBSEQUENT ELECTROCHEMICAL REDUCTION TO Cr(III)
- Chromium,
- ion exchange,
- membrane,
- water-soluble polymer
Copyright (c) 2017 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
This work is focused on the removal of Cr(VI) ions from aqueous solution using water-soluble poly(diallyldimethylammonium chloride), PDDA, coupled to an ultrafiltration membrane of regenerated cellulose and subsequent electrochemical reduction of Cr(VI) to Cr(III). The removal of Cr(VI), using the washing mode, was studied as a function of pH, the molar ratio of polymer:Cr(VI), and the presence of interfering ions. The enrichment mode was used to determine the maximum retention capacity of the polymer, and the release of Cr(VI) and regeneration of the polymer were analysed by sorption-desorption process. Subsequently, the electroanalysis of Cr(VI) was conducted by linear sweep voltammetry and full electrolysis at controlled-potential in acidic media and in the presence of PDDA.
The results show an efficient removal of Cr(VI) (95%, 30 mg/L in the feed) using PDDA at pH 9 and a polymer:Cr molar ratio of 20:1. The retention capacity of the polymer was decreased by the presence of sulphate anions.
The maximum retention capacity of PDDA was 33 mg Cr/g polymer at pH 9. Using the sorption–desorption process, the results indicated that this water-soluble polymer can release Cr(VI) and be regenerated. Finally, the electrolysis of Cr(VI) to Cr(III) was performed successfully in the presence of PDDA.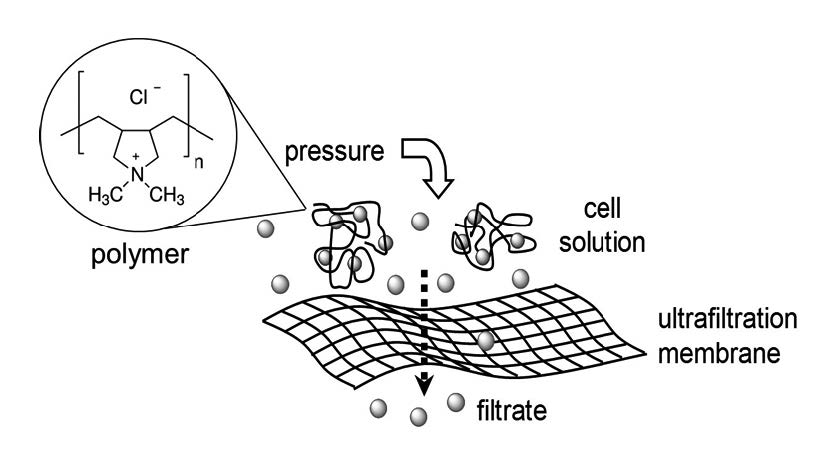
References
- J. Wang, K. Pan, Q. He, B. Cao, J. Hazard. Mater. 244–245, 121, (2013).
- J. Sánchez, B.L. Rivas, Desalination, 279, 338, (2011).
- R. Codd, C.T. Dillon, T. Levina, P.A. Lay, Coordin. Chem. Rev. 216, 537, (2001).
- D. Zhao, A.K. SenGupta, L. Stewart, Ind. Eng. Chem. Res. 37, 4383, (1998).
- M.K. Aroua, F.M. Zuki, N.M. Sulaiman, J. Hazard. Mater. 147, 752, (2007).
- N. Kongsricharoern, C. Polprasert, Water Sci. Technol. 34, 109, (1996).
- L. Alvarado, I.R. Torres, A. Chen, Sep. Purif. Technol. 105, 55, (2013).
- M.S. Bhatti, A.S. Reddy, R.K. Kalia, A.K. Thukral, Desalination, 269, 157, (2011).
- D. Mohan, K.P. Singh, V.K. Singh, Ind. Eng. Chem. Res. 44, 1027, (2005).
- C.A. Kozlowski, W. Walkowiak, Water Res. 36, 4870, (2002).
- B.L. Rivas, E. Pereira, M. Palencia, J. Sánchez, Prog. Polym. Sci. 36, 294, (2011).
- J. Zeng, Q. Guo, Z. Qu-Yang, H. Zhou, H. Chen, Asia-Pac. J. Chem. Eng. 9, 248, (2014).
- S. Chakraborty, J. Dasgupta, U. Farooq, J. Sikder, E. Drioli, S. Curcio, J. Membr. Sci. 456, 139, (2014).
- I. Korus, K. Loska, Desalination, 247, 390, (2009).
- M.A. Barakat, E. Schmidt, Desalination, 256, 90, (2010).
- B.L. Rivas, E.D. Pereira, I. Moreno-Villoslada, Prog. Polym. Sci. 28, 173, (2003).
- S. Yuksel, J. Sánchez, B.L. Rivas, H. Mansilla, J. Yáñez, P. Kochifas, N. Kabay, M. Bryjak, J. Appl. Polym. Sci. 131, 40871, (2014).
- J. Sánchez, B. Butter, B.L. Rivas, L. Basáez, P. Santander, J. Appl. Electrochem. 45, 151, (2015).
- O. Arar, N. Kabay, J. Sánchez, B.L. Rivas, M. Bryjak, C. Peña, Environ. Prog. Sustain. Energy, 33, 918, (2014).
- G. Marcelo, M.P. Tarazona, E. Saiz, Polymer, 46, 2584, (2005).
- H. Dautzenberg, E. Görnitz, W. Jaeger, Macromol. Chem. Physic. 199, 1561, (1998).
- A. Matsumoto, Prog. Polym. Sci. 26, 189, (2001).
- J.D. Roach, D. Tush, Water Res. 42, 1204, (2008).
- J. Sánchez, B.L. Rivas, S. Özgöz, S. Ötles, N. Kabay, M. Bryjak, Polym. Bull. 73, 241, (2016).
- P. Cañizares, A. Pérez, J. Llanos, G. Rubio, Desalination, 223, 229, (2008).
- C.M. Welch, O. Nekrassova, R.G. Compton, Talanta, 65, 74, (2005).
- R.T. Kachoosangi, R.G. Compton, Sensor Actuat. B-Chem. 178, 555, (2013).
- Y. Tapiero, B.L. Rivas, J. Sánchez, J. Chil. Chem. Soc. 59, 2737, (2014).
- O. Kebiche-Senhadji, S. Tingry, P. Seta, M. Benamor, Desalination, 258, 59, (2010).
- J. Sánchez, J. Wolska, E. Yörükoğlu, B.L. Rivas, M. Bryjak, N. Kabay, Desalin. Water Treat. 57, 861, (2016).
- V. Pakade, E. Cukrowska, J. Darkwa, N. Torto, L. Chimuka, Water SA, 37, 529, (2011).
- V. Neagu, S. Mikhalovsky, J. Hazard. Mater. 183, 533, (2010).
- J. Sánchez, A. Bastrzyk, B.L. Rivas, M. Bryjak, N. Kabay, Polym. Bull. 70, 2633, (2013).


