TUNING THE ELECTRONIC, PHOTOPHYSICAL AND CHARGE TRANSFER PROPERTIES OF SMALL D-A MOLECULES BASED ON THIENOPYRAZINE-TERTHIENYLS BY CHANGING THE DONOR FRAGMENT: A DFT STUDY
- Thienopyrazine,
- charge transfer properties,
- donor-acceptor,
- DFT
Copyright (c) 2017 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
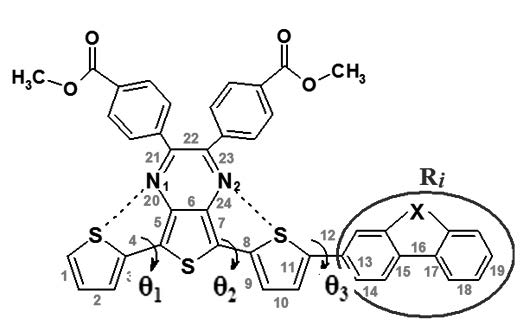
Four acceptor-donor organic conjugated molecules based on thieno[3,4-b]pyrazine-terthienyls were analyzed in order to explore the effect of the donor substituent on their molecular structures, electronic and optical properties. Density Functional Theory (DFT) and Time-Dependent Density Functional Theory (TD/DFT) calculations were carried out employing the B3LYP hybrid functional in combination with the 6-31G(d,p) basis set. The results suggests that the addition of electron-donating substituents to the conjugated molecules can diminish their energy gap value, which is beneficial to the photon harvesting. The lowest-lying absorption spectra of compounds substituted with electron donor groups exhibited a red-shift and a high oscillation factor compared with the unsubstituted molecule. Additionally, the ionization potential (IP), electron affinity (EA), reorganization energy (λ) and open-circuit voltage (Voc) of the molecules were evaluated. According to these values, the molecules show good photovoltaic properties, and efficient charge transfer for hole and electron and balanced charges.
References
- C. Li, M.Y. Liu, N.G. Pschirer, M. Baumgarten, K. Müllen, Chem. Rev. 110, 6817 (2010).
- A.P. Kulkarni, Y. Zhu, S.A. Jenekhe, Macromolecules 38, 1553 (2005).
- J.S. Ha, K. Kim, D.H. Choi, J. Am. Chem. Soc. 133, 10364 (2011).
- E.E. Havinga, W. TenHoeve, H. Wynberg, Synth. Met. 55, 299 (1993).
- G. Brocks, A. Tol, J. Phys. Chem. 100, 1838 (1996).
- Y. Zhang, S.K. Hau, H.-L. Yip, Y. Sun, O. Acton, A. K.-Y. Jen, Chem. Mater. 22, 2696 (2010).
- Y. Zou, A. Najari, P. Berrouard, S. Beaupre, B. Reda Aich, Y. Tao, M. Leclerc, J. Am. Chem. Soc. 132(15), 5330 (2010).
- Q. Zheng, B.J. Jung, J. Sun, H.E. Katz, J. Am. Chem. Soc. 132(15), 5394 (2010).
- J. Zhou, X. Wan, Y. Liu, F. Wang, G. Long, C. Li, Y. Chen, Macromol. Chem. Phys. 212(11), 1109 (2011).
- G. Saranya, P. Kolandaivel and K. Senthilkumar, Mol. Phys. 111, 3036 (2013).
- C. Kitamura, S. Tanaka, Y. Yamashita, Chem. Mater. 8(2), 57 (1996).
- R.L. Schwiderski, S.C. Rasmussen, J. Org. Chem. 78(11), 5453 (2013).
- E. Michael, R.L. Mulholland, S.C. Schwiderski, Rasmussen, Polym. Bull. 69, 291 (2012).
- R.A. Marcus, J. Chem. Phys. 24, 966 (1956).
- R.A. Marcus, Rev. Mod. Phys. 65, 599 (1993).
- Y-Z. Lu, A-M. Ren, J-K. Feng, L.L. Yan, X.Q. Ran, C.S. Chia, J. Phys. Chem. A 112, 12172 (2008).
- B. Priyanka, V. Anusha, K. Bhanuprakash, J. Phys. Chem. C 119(22), 12251 (2015).
- S.F. Nelsen, D.A. Trieber, R.F. Ismagilov, Y. Teki, J. Am. Chem. Soc. 123, (2001) 5684.
- L. Yang, J-K. Feng, A-M. Ren. J. Mol. Struct. THEOCHEM 816, 161 (2007).
- S. Ardo, G. Meyer, J. Chem. Soc. Rev. 38, 115 (2009).
- G.M. Hasselman, D.F. Watson, J.R. Stromberg, D.F. Bocian, D. Holten, J.S. Lindsey, G.J. Meyer, J. Phys. Chem. B 110, 25430 (2006).
- M.C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A.J. Heeger, C. Brabec, J. Adv. Mater. 18, 789 (2006).
- Gaussian 09, Revision C.01, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, J. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al- Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez, J. A. Pople, Gaussian, Inc., Wallingford CT, 2004.
- S.H. Vosko, L. Wilk, M. Nusair, Can. J. Phys. 58(8), 1200 (1980).
- A.D. Becke, J. Chem. Phys. 98(7), 5648 (1993).
- A.D. Becke, Phys. Rev. A. 38(6), 3098 (1988).
- S. Miertus, J. Tomasi, J. Chem. Phys. 65, 239 (1982).
- S. Miertus, E. Scrocco, J. Tomasi, J. Chem. Phys. 1981, 55 (1981).
- J. Casado, R. Ponce-Ortiz, M.C. Ruiz Delgado, V. Hernandez, J.T. Lopez-Navarrete, J. Phys. Chem. B 109, 16616 (2005).
- I.F. Perepichka, D.F. Perepichka. Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics, 2 Volume, John Wiley & Sons, Ltd., Chichester, UK, 2009.
- K. Chitoshi, T. Shoji, Y. Yoshiro, Chem. Mater. 8, 570 (1996).
- F.V. Bolhuis, H. Wynberg, E.E. Havinga, E.W. Mejer, E.G. Stirling, J. Synth. Met. 30, 381 (1989).
- D.D. Kenning, K.A. Mitchell, T.R. Calhoun, M.R. Funfar, D.J. Sattler, S.C. Rasmussen, J. Org. Chem. 67, 9073 (2002).
- M.H. Petersen, O. Hagemann, K.T. Nielsen, M. Jorgensen, F. Krebs. Sol. Energ. Mat. Sol. C. 91, 996 (2007).
- A. Sema-Ozen, C. Atilgan, G. Sonmez, J. Phys. Chem. C 111, 16362 (2007).
- M.C.R. Delgado, V. Hernandez, J.T.L. Navarrete, S. Tanaka, Y. Yamashita, J. Phys. Chem. B 108, 2516 (2004).
- L. Zhang, Q. Zhang, H. Ren, H. Yan, J. Zhang, H. Zhang, J. Gu, Sol. Energ. Mat. Sol. C. 92, 581 (2008).
- Y. Wang, Q. Peng, Z. Li, P. He, B. Li, J. Mol. Model 18, 4291 (2012).
- J.R. Reynolds, A.D. Child, J.P. Ruiz, S.Y. Hong, D.S. Marynick, Macromolecules 26, 2095 (1993).
- M.A. De Oliveira Helio, A. Duarte Pernaut, J.M. Wagner, B. De Almeida, J. Phys. Chem. A 104, 8256 (2000).
- C.J. Brabec, S. Gowrisanker, J.J.M. Halls, D. Laird, S. Jia, S.P. Williams, Adv Mater. 22, 3839 (2010).
- C.J. Brabec, N.S. Sariciftci, J.C. Hummelen, Adv. Funct. Mater. 11, 15 (2001).
- G. Dennler, M.C. Scharber, C. Brabec, Adv. Mater. 21, 132 (2009).
- J.W. Chen, Y. Cao, Acc. Chem. Res. 42, 1709 (2009).
- Zaumseil J., Sirringhaus H, Chem Rev. 107, 1296 (2007).
- R. Schmidt, J.H. Oh, Y.-S. Sun, M. Deppisch, A.-M. Krause, K. Radacki, H. Braunschweig, M. Konemann, P. Erk, Z. Bao, F. Wurthner, J. Am. Chem. Soc. 131, 6215 (2009).
- C.R. Newman, C.D. Frisbie, D.A. Da Silva Filho, J.-L. Bredas, P.C. Ewbank, K.R. Mann, Chem. Mater. 16, 4436 (2004).
- G. Saranya, P. Kolandaivel, K. Senthilkumar, Mol. Phys. 111(20), 3036 (2013).
- E. Bundgaard, F.C. Krebs. Sol. Energ. Mat. Sol. C. 91, 954 (2007).
- M. Helgesen, S.A. Gevorgyan, C.K. Frederik, Chem. Mater. 21, 4669 (2009).
- J.P. Nietfeld, R.L. Schwiderski, T.P. Gonnella, S.C. Rasmussen, J. Org. Chem. 76, 6383 (2011).
- S.C. Rasmussen, J.S. Daniel, K.A. Mitchell, J. Maxwell, J. Lumin. 109, 111 (2004).
- E. Wang, M. Wang, L. Wang, C. Duan, J. Zhang, W. Cai, C. He, H. Wu, Y. Cao, Macromol. 42, 4410 (2009).


