SYNTHESIS, CHARACTERIZATION AND CRYSTAL STRUCTURE OF A NOVEL DECAVANADATE SALT, [V0.50(H2O)5]2[H2(V10O28)]·4(H2O)
- Decavanadate,
- V(V) and V(IV) units,
- chinhydrone,
- single crystal,
- water-bridged dimer
Copyright (c) 2017 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
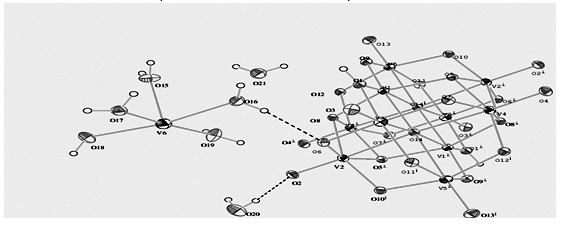
A novel decavanadate salt, [V0.50(H2O)5]2[H2(V10O28)]·4(H2O), was obtained by the reaction of NH4VO3 with chinhydrone in an acidic medium. The title compound was characterized in the solid and solution phases by FT-IR, single crystal X-ray diffraction, voltammetry, UV-Vis., EPR and NMR measurements. The crystal data of the compound are: H30O42V11, triclinic, Pī, a = 8.4906 (4) Å, b = 10.4236 (5) Å, c = 11.2861 (6) Å, α = 68.490 (4)°, β = 87.251 (4)°, γ = 67.145 (4)°, V = 851.11 (8) Å3, and Z = 1. In the structure, [H2(V10O28)]4- anion is bound via hydrogen bonds to the dimer state of [V0.50(H2O)5]2+ cations and the water molecules. At this dimer moiety, it has been thought that the oxygen atoms of two water molecules form the bridges between two V(IV) center ions. The presence of high spin V(IV) (I = 7/2) in the compound was verified by EPR measurements. In addition, decavanadate unit gave a shoulder at about 400 nm on the UV-Vis. spectrum of the compound. Moreover, at the FT-IR spectrum, the symmetric and asymmetric stretching vibrations of the bridging V–O–V units were observed. On the cyclic voltammogram of the compound, two reduction peaks (V(V)-V(IV) and V(IV)-V(II) reduction steps, respectively) at −1.03 and −1.35 V were observed at the cathodic scan; however, one oxidation peak (V(II)-V(III) oxidation step) at −1.22 V showed at the anodic scan. NMR results also support that the synthesized salt includes the coordinated water molecules and hydroxo groups.
References
- Y. Shechter, A. Shisheva, Endeavour, 17, 27, (1993).
- D. Rehder, Coord. Chem. Rev., 182, 297, (1999).
- A. Butler, J.V. Walker, Chem. Rev., 93, 1937, (1993).
- D. Rehder, Angew. Chem. Int. Ed. Engl., 30, 148, (1994).
- L.C.W. Baker, D.C. Glick, Chem. Rev., 98, 3, (1998).
- M. Weyand, H.J. Hecht, M. Kiez, M.F. Liaud, H. Vilter, D. Schomburg, J. Mol. Biol., 595, 293, (1999).
- A. Messerschdt, L. Prade, R. Wever, Biol. Chem., 378, 309, (1997).
- D. del Rio, A. Galindo, J. Tejedo, F.J. Bedoya, A. Ienco, C. Mealli, Inorg. Chem. Commun., 3, 32, (2000).
- H. Sigel, A. Sigel (Eds.), Metal Ions in Biological Systems, vol. 31, Marcel Dekker, New York, 1995.
- A. Butler, M.J. Clague, G. E. Meister, Chem. Rev., 94, 625, (1994).
- D.C. Crans, Comment. Inorg. Chem., 16, 35, (1994).
- A. Butler, C.J. Carrano, Coordin. Chem. Rev., 109, 61, (1991).
- N.D. Chasteen (Ed.), Vanadium in Biological Systems, Kluwer Academic Publishers, Dordrecht, 1990.
- S. Ramanadham, J.J. Mongold, R.W. Browsey, G.H. Cros, J.H. McNeill, Am. J. Physiol., 257, H904, (1989).
- V.G. Yuen, C. Orvig, J.H. McNeill, Can. J. Physiol. Pharmacol., 73, 55, (1995).
- M. Halberstam, N. Cohen, P. Shlimovich, L. Rossetti, H. Shamoon, Diabetes, 45, 659, (1996).
- V. Badmaev, S. Prakash, M. Majeed, J. Altern. Complement. Med., 5, 273, (1999).
- M. Aureliano, D.C. Crans, J. Inorg. Biochem., 103, 536, (2009).
- M.S. Whittingham, J. Electrochem. Soc., 123, 315, (1978).
- A. Vejiux, P. Courtini, J. Solid State Chem., 63, 179, (1996).
- Y. Ueda, Chem. Mater., 10, 2653, (1998).
- H. Yoshihito, Coordin. Chem. Rev., 255, 2270, (2011).
- D. M. Tiago, V. Laizé, M. L. Cancela, M. Aureliano, Cell Biology and Toxicology, 24, 253, (2008).
- H.T. Evans, Inorg. Chem., 5, 967, (1966).
- I. Mestiri, I. Nagazi, A. Haddad, Journal of the Tunisian Chemical Society, 17, 128, (2015).
- U. Lee, Y.-H. Jung, H.-C Joo, Acta Cryst. E, 59, i72, (2003).
- T. Higami, M. Hashimoto, S. Okeya, Acta Cryst. C, 58, i144, (2002).
- G. Maciejewska, M. Nosek, T. Głowiak, J. Starosta, M. Cieslak-Golonka, Polyhedron, 22, 1415, (2003).
- D. Omanović, M. Branica, Croat. Chem. Acta, 71, 421, (1998).
- STOE & Cie, X-AREA (Version 1.18) and X-RED32 (Version 1.04), STOE & Cie, Darmstadt, Germany, 2002.
- G.M. Sheldrick, Acta Crystallogr. A, 64, 112, (2008).
- L.J. Farrugia, J. Appl. Crystallogr., 30, 565, (1997).
- A.L. Spek, Acta Crystallogr. D, 65, 148, (2009).
- L.J. Farrugia, J. Appl. Crystallogr., 32, 837, (1999).
- V.D. Kassabova-Zhetcheva, L.P. Pavlova, Turk. J. Chem., 35, 215, (2011).
- M. Shahid, P.K. Sharma, Anjuli, S. Chibber, Z.A. Siddiqi, J. Clust. Sci., 25,1435, (2014).
- Z.A. Siddiqi, Anjuli, P.K. Sharma, M. Shahid, M. Khalid, A. Siddique, S. Kumar, J. Mol. Struct., 1029, 86, (2012).
- Y.T. Chua, P.C. Stair, I.E. Wachs, J. Phys. Chem. B, 105, 8600, (2001).
- S.B. Etcheverry, P.A.M. Williams, D.A. Barrio, V.C. Sálice, E.G. Ferrer, A.M. Cortizo, J. Inorg. Biochem., 80, 169, (2000).
- O.M. Yaghi, H. Li, T.L. Groy, J. Am. Chem. Soc., 118, 9096, (1996).
- R.L. Frost, S.J. Palmer, D.A. Henry, R. Pogson, J. Raman Spectrosc., 42, 1447, (2011).
- R.L. Frost, M.J. Dickfos, J. Raman Spectrosc., 38, 1516, (2007).
- J.A. Gomes, D.L. Cocke, M.A. Mahmud, H. Moreno, E. Peterson, M.Y. Mollah, J.R. Parga, ECS Transactions, 6, 29, (2008).
- R.C. Maurya, D. Sutradhar, M.H. Martin, S. Roy, J. Chourasia, A.K. Sharma, P. Vishwakarma, Arabian Journal of Chemistry, 8, 78, (2015).
- G. Arrambide, D.A. Barrio, S.B. Etcheverry, D. Gambino, E.J. Baran, Biol. Trace Elem. Res., 136, 241, (2010).
- A. Müller, E. Diemann, C.K. Jørgensen, Struct. Bond, 14, 23, (1973).
- M. Tarnai, Oxidation of transition metal complexes with 3,7-diazabicyclo[3.3.1]nonane-derived ligands, PhD Thesis, Ruprecht- Karls-Universität Heidelberg, Ungarn, 2006.
- L. Pajdowski, Chem. Zvesti, 19, 192, (1965).
- S.M. Lewis, D.D. Thomas, Biochemistry, 25, 4615, (1986).
- S. Ramos, R.O. Duarte, J.J.G. Mourac, M. Aureliano, Dalton T., 7985, (2009).
- S. Bhattacharya, T. Ghosh, Indian J. Chem. A, 38, 601, (1999).
- N.A. Mangalam, M.R.P. Kurup, Spectrochim. Acta A, 71, 2040, (2009).
- Y. Israel, L. Meites, in Encyclopedia of Electrochemistry of the Elements, A.J. Bard (Ed.), Vol. VII, Ch. 2, Marcel Dekker, New York, 1976; pp. 293- 466.
- G. Nowogrocki, E. Baudrin, S. Denis, M. Touboul, Eur. J. Solid State Inorg. Chem., 34, 1011, (1997).
- E. Rakovský, L. Zúrková, J. Marek, Cryst. Res. Technol., 36, 339, (2001).
- N. Pavlovic, J. Prevost, A. Spasojevic-de Bire, Cryst. Growth Des., 11, 3778, (2011).
- A. Najafi, J.T. Mague, M. Mirzaei, J. Iran. Chem. Soc., 13, 773, (2016).
- L. Zhang, Z. Chen, Chemical Physics Letters, 345, 353, (2001).
- D.C. Crans, A.S. Tracey, ACS Symposium Series, 711, 2, (1998).
- C. Pretorius, A solution and solid state study of vanadium complexes, Magister Scientiae Thesis, University of the Free State, Bloemfontein, 2012.
- J. Burgess (Ed.), Inorganic Reaction Mechanisms, Vol. 1, Part 1, Ch. 5, The Chemical Society, Burlington House, London, 1971; p. 56.
- http://www.chemicalbook.com/ChemicalProductProperty_DE_ CB9292163.htm (Accessed 18 February 2016).
- Z.A. Siddiqi, P.K. Sharma, M. Shadid, M. Khalid, S. Kumar, J. Mol. Struct., 994, 295, (2011).
- A. F. A. Peacock, M. Melchart, R. J. Deeth, A. Habtemariam, S. Parsons, P. J. Sadler, Chem. Eur. J., 13, 2601, (2007).
- http://www.iza-online.org/synthesis/VS_2ndEd/NMR.htm (Accessed 05 November 2016).


