SYNTHESIS OF CARBOXYL-MODIFIED Fe3O4@SiO2 NANOPARTICLES AND THEIR UTILIZATION FOR THE REMEDIATION OF CADMIUM AND NICKEL FROM AQUEOUS SOLUTION
- Iron oxide nanoparticles,
- adsorption,
- heavy metals removal,
- co-precipitation method,
- malic acid
Copyright (c) 2017 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
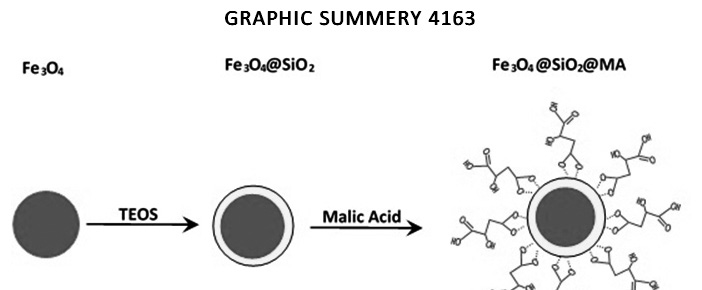
In the present study, general and versatile method for the functionalization of silica coated Fe3O4 nanoparticles by surface carboxylic group have been established. We have shown that malic acid functionalized magnetic nanoparticles (MA-MNPs) are an effective sorbent material for toxic metals such as cadmium and nickel. This magnetic sorbent was characterized by X-ray diffraction, scanning electron microscope and infra-red spectra. The adsorption of all studied metal ions onto Malic acid functionalized magnetic nanoparticles was found to be dependent on pH. Batch adsorption equilibrium was very fast under optimum conditions and maximum monolayer capacity, Qm, obtained from Langmuir isotherm for Cd2+ and Ni2+ were 81.627 and 63.995mgg−1, respectively at 25◦C. Adsorption data were fitted well to Langmuir and Freundlich isotherm (R2 ≈ 0.99). The malic acid grafted on Fe3O4@SiO2 enhanced the adsorption capacity because of the complexing abilities of the multiple hydroxyl and carboxyl groups with metal ions.
References
- Badruddoza, A. Z. M., Shawon, Z. B. Z., Tay, W. J. D., Hidajat, K., & Uddin, M. S. (2013). Fe 3 O 4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydrate polymers,91(1), 322-332.
- Ozay, O., Ekici, S., Baran, Y., Aktas, N., & Sahiner, N. (2009). Removal of toxic metal ions with magnetic hydrogels. Water Research, 43(17), 4403-4411.
- Adeli, M., Yamini, Y., & Faraji, M. (2012). Removal of copper, nickel and zinc by sodium dodecyl sulphate coated magnetite nanoparticles from water and wastewater samples. Arabian Journal of Chemistry.
- Stafiej, A., & Pyrzynska, K. (2007). Adsorption of heavy metal ions with carbon nanotubes. Separation and Purification Technology, 58(1), 49-52.
- Jian, X., Wu, B., Wei, Y., Dou, S. X., Wang, X., He, W., & Mahmood, N. (2016). Facile Synthesis of Fe3O4/GCs Composites and Their Enhanced Microwave Absorption Properties. ACS applied materials & interfaces, 8(9), 6101-6109.
- Oliveira, L. C., Petkowicz, D. I., Smaniotto, A., & Pergher, S. B. (2004). Magnetic zeolites: a new adsorbent for removal of metallic contaminants from water. Water Research, 38(17), 3699-3704.
- Hu, J., Lo, I., & Chen, G. (2004). Removal of Cr (VI) by magnetite. Water Science & Technology, 50(12), 139-146.
- Hu, J., Chen, G., & Lo, I. M. (2005). Removal and recovery of Cr (VI) from wastewater by maghemite nanoparticles. Water Research, 39(18), 4528-4536.
- Yavuz, C. T., Mayo, J. T., William, W. Y., Prakash, A., Falkner, J. C., Yean, S., & Colvin, V. L. (2006). Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science, 314(5801), 964-967.
- Hai, B., Wu, J., Chen, X., Protasiewicz, J. D., & Scherson, D. A. (2005). Metal-ion adsorption on carboxyl-bearing self-assembled monolayers covalently bound to magnetic nanoparticles. Langmuir, 21(7), 3104-3105.
- Hu, J., Lo, I. M., & Chen, G. (2007). Comparative study of various magnetic nanoparticles for Cr (VI) removal. Separation and Purification Technology, 56(3), 249-256.
- Chang, Y. C., & Chen, D. H. (2005). Preparation and adsorption properties of monodisperse chitosan-bound Fe 3 O 4 magnetic nanoparticles for removal of Cu (II) ions. Journal of Colloid and Interface Science, 283(2), 446-451.
- Liu, J. F., Zhao, Z. S., & Jiang, G. B. (2008). Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environmental science & technology, 42(18), 6949-6954.
- Yantasee, W., Warner, C. L., Sangvanich, T., Addleman, R. S., Carter, T. G., Wiacek, R. J., ... & Warner, M. G. (2007). Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles. Environmental science & technology, 41(14), 5114-5119.
- Zhou, Y. T., Nie, H. L., Branford-White, C., He, Z. Y., & Zhu, L. M. (2009). Removal of Cu 2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid. Journal of colloid and interface science, 330(1), 29-37.
- Gong, J., Chen, L., Zeng, G., Long, F., Deng, J., Niu, Q., & He, X. (2012). Shellac-coated iron oxide nanoparticles for removal of cadmium (II) ions from aqueous solution. Journal of Environmental Sciences, 24(7), 1165- 1173.
- Wang, J., Zheng, S., Shao, Y., Liu, J., Xu, Z., & Zhu, D. (2010). Amino-functionalized Fe 3 O 4@ SiO 2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. Journal of Colloid and Interface Science, 349(1), 293-299.
- Mahmoud, M. E., Abdelwahab, M. S., & Fathallah, E. M. (2013). Design of novel nano-sorbents based on nano-magnetic iron oxide–bound-nano-silicon oxide–immobilized-triethylenetetramine for implementation in water treatment of heavy metals. Chemical Engineering Journal, 223, 318-327.
- Li, Y. S., Church, J. S., & Woodhead, A. L. (2012). Infrared and Raman spectroscopic studies on iron oxide magnetic nano-particles and their surface modifications. Journal of Magnetism and Magnetic Materials, 324(8), 1543-1550.
- Naiya, T. K., Bhattacharya, A. K., & Das, S. K. (2009). Adsorption of Cd(II) and Pb(II) from aqueous solutions on activated alumina. Journal of Colloid and Interface Science, 333, 14–26.
- Nassar, N. N. (2011). Kinetics, equilibrium and thermodynamic studies on the adsorptive removal of nickel, cadmium and cobalt from wastewater by superparamagnetic iron oxide nanoadsorbents. Canadian Journal of Chemical Engineering, 9999, 1–8.
- Singh, S., Barick, K., & Bahadur, D. (2011). Surface engineered magnetic nanoparticles for removal of toxic metal ions and bacterial pathogens. Journal of Hazardous Material, 192, 1539–1547.
- Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of American Chemical Society, 40, 1361–1403.
- Ng, C., Losso, J. N., Marshall, W. E., & Rao, R. M. (2002). Freundlich adsorption isotherms of agricultural by-product-based powdered activated carbons in a geosmin–water system. Bioresource technology, 85(2), 131- 135.


