CERIUM (IV) AMMONIUM NITRATE (CAN) AS A CATALYST IN WATER: A SIMPLE, PROFICIENT AND GREEN APPROACH FOR THE SYNTHESIS OF TETRAHYDROPYRIMIDINE QUINOLONES
- Tetrahydropyrimidine quinolones,
- One-pot,
- Multi-component,
- Green chemistry,
- Cerium ammonium nitrate
Copyright (c) 2017 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
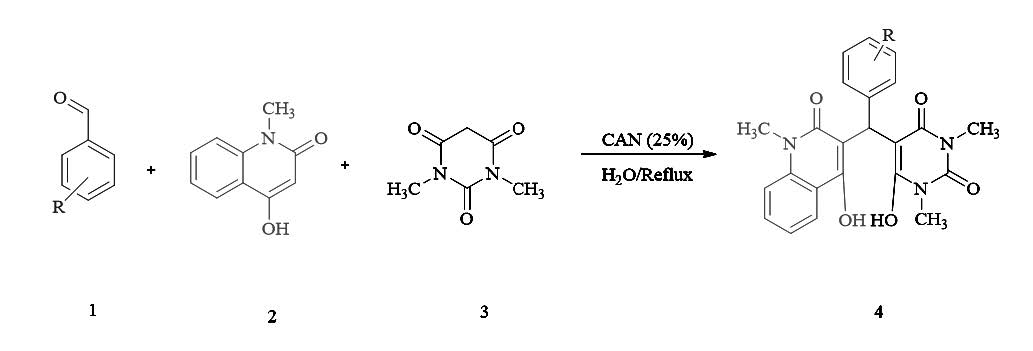
We report here a facile, mild and efficient synthesis of functionalized tetrahydropyrimidine quinolones via one pot three-component condensation of aromatic aldehydes, N,N-dimethyl barbituricacid (barbituricacid), 4-hydroxy-1-methylquinolin-2(1H)-one and using a cerium ammonium nitrate (CAN) as a green catalyst in aqueous media-water (as a green ideal solvent) is described. The significant advantages of this protocol are short reaction time, simple work up procedure and reduced environmental impact, wide substrate scope; generally very good to excellent yields and starting materials are inexpensive and commercially available. The structures of these compounds were established on the basis of IR, 1H NMR, 13C NMR spectra.
References
- (a) R. V. A. Orru, M. de Greef, Synthesis (2003) 1471. (b) D. Tejedor, D. Gonza´lez-Cruz, A. Santos-Expósito, J. J. Marrero-Tellado, P. de Armas, F. García-Tellado, Chem. Eur. J. 11 (2005) 3502.
- (a) A. Dömling, Chem. Rev. 106 (2006) 17; (b) B. B. Toure, D. G Hall, Chem. Rev. 109 (2009) 4439.
- E. Verònica, V. Mercedes, J. C. Menéndez, Chem. Soc. Rev. 39 (2010) 4402.
- J. Zhu, H. Bienaymé; 2nd Ed.; Multicomponent Reactions; Wiley-VCH: Weinheim, 2005.
- A. Dandia, S. L. Gupta, S. Bhaskaran, Eur. Chem. Bull. 2 (2013) 836.
- Z. Zhou, Y. Zhang, J. Chil. Chem. Soc. 60 (2015) 2992.
- M. Zakeri, M. M. Nasef, E. Abouzari-Lotf, A. Moharami, M. M. Heravi, J. Ind. Eng. Chem. 29 (2015) 273.
- M. Zakeri, M. M. Nasef, E. Abouzari-Lotf, J. Mol. Liq. 199 (2014) 267.
- B. S.Holla, B. Kalluraya, K. R. Sridhar, E. Drake, L. M. Thomas, K. K. M. Bhandary, J. Levine, Eur. J. Med. Chem. 29 (1994) 301.
- P. Molina, E. Aller, A. Lorenzo, P. López-Cremades, I. Rioja, A. Ubeda, M. C. Terencio, M. J. Alcaraz, J. Med. Chem. 44 (2001) 1011.
- A. H. Shamroukh, M. E. A. Zaki, E. M. H. Morsy, F. M. Abdel-Motti, F. M. Abdel-Megeid, Arch. Pharm. 340 (2007) 236.
- J. A. Valderrama, P. Colonelli, D. Vásquez, M. F. González, J. A. Rodríguez, C. Theoduloz, Bioorg. Med. Chem. 16 (2008) 10172.
- O. Bruno, C. Brullo, A. Ranise, S. Schenone, F. Bondavalli, E. Barocelli, V. Ballabeni, M. Chiavarini, M. Tognolini, M. Impicciatore, Bioorg. Med. Chem. Lett. 11 (2001) 1397.
- N. R. Kamdar, D. D. Haveliwala, P. T. Mistry, S. K. Patel, Eur. J. Med. Chem. 45 (2010) 5056.
- (a) S. S. Chobe, B. S. Dawane, K. M. Tumbi, P. P. Nandekar, A. T. Sangamwar, Bioorg. Med. Chem. Lett. 22 (2012) 7566; (b) M. T. Di Parsia, C. Suarez, M. J. Vitolo, V. E. Marquez, B. Beyer, C. Urbina, I. Hurtado, J. Med. Chem. 24 (1981) 117.
- 16.- M. N. Elinson, A. I. Ilovaisky, V. M. Merkulova, T. A. Zaimovskaya, G. I. Nikishin Mendeleev Commun. 21 (2011) 122.
- A. Rahmati, Z. Khalesi, Tetrahedron 68 (2012) 8472.
- (a) C. J. Chan, T. H. Li, 1nd Ed.; Organic Reactions in Aqueous Media; Wiley New: York, NY, 1997; (b) A. Grieco, Organic Synthesis in Water; Blackie Academic and Professional, 1998; (c) A. Chanda, V. V. Fokin, Chem. Rev. 109 (2009) 725; (d) M. C. Pirrung, K. D. Sarma, J. Am. Chem. Soc. 126 (2004) 444.
- K. Kandhasamy, V. Gnanasambandam, Curr. Org. Chem. 13 (2009) 1820.
- C. K. Z. Andrade, L. M. Alves, Curr. Org. Chem. 9 (2005) 195.
- (a) V. Nair, A. Deepthi, Chem. Rev. 107 (2007) 1862; (b) V. Nair, S. B. Panicker, L. Nair G. George, A. Augustine Synlett (2003) 156.
- V. Shivaji, M. N. V. More, S. C. Yao, Green Chem. 8 (2006) 91.
- T. D. V. Nair, S. M Kishor, Tetrahedron Lett. 46 (2005) 3217.
- Sh. Ko, Ch. F. Yao, Tetrahedron 62 (2006) 7293.
- H. J. Wang, L. Hui, Zh. Zhang, ACS Comb. Sci. 13 (2011) 181.
- (a) P. K. Tapaswi, C. Mukhopadhyay Arkivoc (2011) 287; (b) V. Sridharan, J. C. Menendez, Org. Lett. 10 (2008) 4303; (c) M. Y. Chang, T. C. Wu, C. C. Y. Lin, Y. Hung Tetrahedron Lett. 47 (2006) 8347.
- (a) K. U. Sadek, A. Alnajjar, R. A. Mekheimer, N. K. Mohamed, H. A. Mohamed, Green and Sustainable Chem. 1 (2011) 92; (b) A. Mekhalifa, R. H. Mutter, W. B. Chen, Tetrahedron 62 (2006) 5617.


