STABILITY INDICATING RP-HPLC METHOD FOR SIMULTANEOUS DETERMINATION OF CIPROFLOXACIN AND DEXAMETHASONE IN BINARY COMBINATION
- Liquid Chromatographic,
- Ciprofloxacin,
- Dexamethasone,
- Degradation Products,
- ICH Guidelines
Copyright (c) 2017 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
A simple and isocratic HPLC method with stability indicating nature was developed and then subsequently validated for simultaneous determination of ciprofloxacin and dexamethasone in pharmaceutical formulations, human serum and urine. Best chromatographic separations were attained within run time of 10 minutes using C8 as stationary phase and mixture of phosphate buffer and methanol (41:59 v/v) as mobile phase. The mobile phase was flowed at 1.5 mL min-1 with detection of both the analytes at 270 nm using photodiode array detector. Validation of the method was accomplished using specificity, linearity, accuracy, precision, robustness, LOD and LOQ. The method was found linear from 3-21 mg mL-1 for ciprofloxacin (r2 ≥ 0.999) and 1-7 mg mL-1 for dexamethasone (r2 ≥ 0.999). The %age recoveries of ciprofloxacin in spiked human urine and serum were ≥99% and ≥85% respectively, while for dexamethasone they were ≥97% in both matrices. The method proficiently separated the peaks of ciprofloxacin and dexamethasone from all types of interfering substances including degradation products/impurities with purity index ≥ 0.9998. The method thus was stability-indicating and can be employed for simultaneous analysis of ciprofloxacin and dexamethasone in complex matrices involving multiple components in the mixture.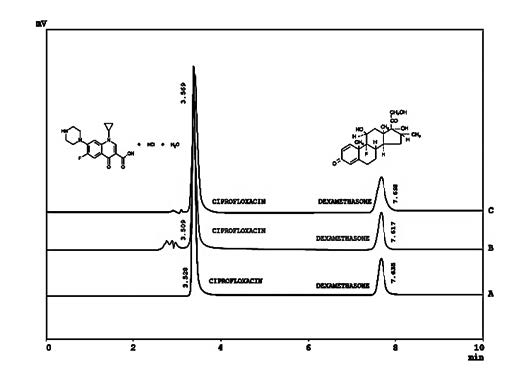
References
- Reynolds, J.E.F.; Martindale, The Extra Pharmacopoeia, 36th ed., Pharmaceutical Press: London; 2009; p. 243 and 1526.
- Predrag, S.; Andreja, M.; Radosav, P.; Sinia, A.; and Valentina, M. Ruggedness testing of an HPLC method for the determination of ciprofloxacin. J. Serb. Chem. Soc. 2005, 70 (7), 979–986.
- Marika, K.; Kimiko, T.; Tsutomu, K.; Koichi, N.; and Shigeyuki, N. Determination of ciprofloxacin in plasma and urine by HPLC with ultraviolet detection. J. Clinical Chemistry. 1998, 44 (6), 1251-1255.
- Mohsen, A.; Mahnaz, K.; and Abbas, S. Simple high performance liquid chromatographic method for determination of ciprofloxacin in human plasma. Iran. J. Pharm. Res. 2004, 2, 99-101.
- Zafar, A. k.; Jamshaid, A. K.; Imran, K.; Ghulam, S. K; Bilal, M.; Tahir, M. K. The development and validation of HPLC-UV method for analysis of ciprofloxacin in serum and aqueous humour. J. Arch. Pharm. Prac. 2011, 2 (3), 116-122.
- Ranjit, S.; Mukesh, M.; Shailendra, K. S.; Shubhini, S.; and Ram C. G. Simultaneous estimation of ciprofloxacin hydrochloride, ofloxacin, tinidazole and ornidazole by reverse phase high performance liquid chromatography. Eur. J. Anal. Chem. 2009, 4(2), 161-167.
- Espinosa, M. A.; Muñoz, A.; González, G. D. HPLC determination of ciprofloxacin, cloxacillin, and ibuprofen drugs in human urine samples. J. Sep. Sci., 2006, 29(13), 1969-76.
- Sebastian, J. M.; Virginia, H. S.; and James, D. D. 1986. Determination of norfloxacin and ciprofloxacin concentrations in serum and urine by high-pressure liquid chromatography. J. Antimicr. Agent. chemo. 1986, 30 (2), 325-327.
- Cazedey, E.C.L.; Perez, D.P.; Perez, J.P.; Salgado, H.R.N. A high performance liquid chromatographic assay for ciprofloxacin hydrochloride ophthalmic solution. Chromatographia. 2009, 69, 241-244.
- Cazedey, E.C.L.; Bonfilio, R.; Araújo, M.B.; Salgado, H.R.N. A first-derivative spectrophotometric method for the determination of ciprofloxacin hydrochloride in ophthalmic solution. Phys. Chem. 2012, 2(6), 116-122.
- Cazedey, E.C.L.; Salgado, H.R.N. Spectrophotometric determination of ciprofloxacin hydrochloride in ophthalmic solution. Adv. Anal. Chem., 2012, 2(6), 74-79.
- Rajia, S. N.; Yeakuty, M. J.; and Kumar, B. S. Development of an assay method for simultaneous determination of ciprofloxacin and naproxen by UV Spectrophotometric method. J. Pharm. Sci. 2011, 4(1), 84-90.
- Durmus, Z.; Canel, E.; and Kiliq, E. Spectrofluorimetric assay of ciprofloxacin hydrochloride in tablets. J. Anal. Quant. Cytol. Histol. 2005, 27(3), 162-6.
- Basavaiah, K.; and Nagegowsa, P. Titrimetric and spectrophotometric assay methods for ciprofloxacin in pharmaceuticals based on neutralization reaction. J. Natio. Acad. Sci. Lett. 2006, 29(5), 189-194.
- Iqbal, M. S.;, Shad, M. A.; Ashraf, M. W.; Bilal, M.; and Saeed, M. Development and validation of an HPLC method for the determination of dexamethasone, dexamethasone sodium phosphate and chloramphenicol in presence of each other in pharmaceutical preparations. Chromatographia, 2006, 64(4), 219-222.
- Huetos, O.; Ramos, M.; Martín, D. P. M.; San, A. M.; and Reuvers, T. B. Determination of dexamethasone in feed by TLC and HPLC. Analyst. 1999, 124 (11), 1583-7.
- Mallinson, E. T.; Dreas, J. S.; Roger, T.; Wilson, A.; and Henry, C. Determination of dexamethasone in liver and muscle by liquid chromatography and gas chromatography/mass spectrometry. J. Agric. Food Chem. 1995, 43 (1), 140–145.
- Chen, Q.; Zielinski, D.; Chen, J.; Koski, A.; Werst, D.; and Nowak, S. A. Validated, stability-indicating HPLC method for the determination of dexamethasone related substances on dexamethasone-coated drug-eluting stents. J. Pharm. Biomed. Anal. 2008, 48 (3), 732-738.
- Gallego, J. M. L.; and Arroyo, J. P. Simultaneous determination of dexamethasone and trimethoprim by liquid chromatography. J. Pharm. Biomed. Anal. 2002, 30, 1255-1261.
- Urban, M. C. C.; Mainardes, R. M.; Gremião, M. P. D. Development and validation of HPLC method for analysis of dexamethasone acetate in microemulsions. Braz. J. Pharma. Sci. 2009, 45 (1), 87-92.
- Hyung, W. K. M. D.; Amico, D. J. D.; Korean M. D. Determination of dexamethasone sodium phosphate in vitreous by high performance liquid chromatography. J. ophthalmol. 1995, 9, 79-83.
- Rele, R. V.; and Warkar, C. B. Simultaneous determination of ciprofloxacin hydrochloride and dexamethasone in ophthalmic solution by reversed phase high performance liquid chromatography. Asian J. Res. Chem. 2010, 3, 673-677.
- Prakash, K.; Sireesha, K. R. Simultaneous determination of ciprofloxacin hydrochloride and dexamethasone sodium phosphate in eye drops by HPLC. E-J. Chem. 2012, 9(3), 1077-1084.
- Razzaq, S. N.; Mariam, I.; Khan, I. U.; Ashfaq, M. Development and validation of liquid chromatographic method for gatifloxacin and ketorolac tromethamine in combined dosage form. J. Liq. Chromat. Rel. Technol. 2012, 35, 651-661.
- Razzaq, S. N.; Ashfaq, M.; Khan, I. U.; Mariam, I. Development and validation of liquid chromatographic method for moxifloxacin and ketorolac tromethamine in combined dosage form. Quim Nova. 2012, 35, 1216-1221.
- Razzaq, S. N.; Ashfaq, M.; Khan, I. U.; Mariam, I. Stability indicating HPLC method for the simultaneous determination of ofloxacin and ketorolac tromethamine in pharmaceutical formulations. Anal. Meth. 2012, 4, 2121-2126.
- Razzaq, S. N.; Ashfaq, M.; Khan, I. U.; Mariam, I. Development and validation of liquid chromatographic method for naproxen and esomeprazole in binary combination. J. Chil. Chem. Soc. 2012, 57, 1456- 1459.
- Razzaq, S. N.; Ashfaq, M.; Mariam, I.; Khan, I. U.; Razzaq, S. S. Simultaneous RP-HPLC determination of sparfloxacin and dexamethasone in pharmaceutical formulations. Braz. J. Pharm. Sci. 2013, 49, 301-309.
- Khan, I. U.; Ashfaq, M.; Razzaq, S. N.; Mariam, I. Simultaneous determination of piroxicam and paracetamol in pharmaceutical formulations using stability indicating HPLC method. J. Liq. Chromat. Rel. Technol. 2013, 36, 1437-1450.
- Ashfaq, M.; Ahmad, H.; Khan, I. U.; Mustafa, G. LC determination of rosuvastatin and ezetimibe in human plasma. J. Chil. Chem. Soc. 2013, 58, 2177-2181.
- Khan, I. U.; Razzaq, S. N.; Mariam, I.; Ashfaq, M.; Razzaq, S. S. Stability indicating RP-HPLC method for simultaneous determination of gatifloxacin and flurbiprofen in binary combination. Quim Nova, 2014, 37, 349-354.
- Ashfaq, M.; Akhtar, T.; Mustafa, G.; Danish, M.; Razzaq, S. N.; Nazar, M. F. Simultaneous estimation of rosuvastatin and amlodipine in pharmaceutical formulations using stability indicating HPLC method. Braz. J. Pharm. Sci. 2014, 50, 629-638.
- Saleem, A.; Anwar, S.; Hussain, T.; Ahmad, R.; Mustafa, G.; Ashfaq, M.; Simultaneous Determination of Acetaminophen, Pamabrom and Pyrilamine Maleate in Pharmaceutical Formulations Using Stability Indicating HPLC Assay Method. J. Mex. Chem. Soc. 2015, 59, 93-98.
- John, P.; Azeem, W.; Ashfaq, Khan, I. U.; Razzaq, S. N.; Khan, S. U.D. Stability-indicating RP-HPLC method for simultaneous determination of methoxsalen and p-aminobenzoic acid in binary combination. Bull. Chem. Soc. Ethiop. 2015, 29, 27-39.
- John, P.; Azeem, W.; Ashfaq, Khan, I. U.; Razzaq, S. N. Stability-indicating RP-HPLC method for simultaneous determination of piroxicam and ofloxacin In binary combination. Pak. J. Pharm. Sci., 2015, 28, 1713- 1721.
- Razzaq, S. N.; Ashfaq, M.; Khan, I. U.; Mariam, I.; Razzaq, S. S. Azeem, W. Simultaneous determination of dexamethasone and moxifloxacin in pharmaceutical formulations using stability indicating HPLC assay method. Arab. J. Chem. In press. http://dx.doi.org/10.1016/j.arabjc.2014.11.016.
- ICH (Q2B). Note for guidance on validation of analytical procedures: methodology. International conference on Harmonization, IFPMA, Geneva 1996.


