- chiral separation,
- Captisol®,
- capillary electrophoresis,
- cyclodextrines,
- pharmaceutical analysis
Copyright (c) 2017 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Enantioseparation of nine extensively used chiral pharmaceutical substances from different pharmacological classes and with different structural characteristics has been investigated by capillary zone electrophoresis using Captisol® (sulfobuthylether-β-cyclodextrin sodium salt) as chiral selector. The influence on the chiral separation of several parameters including pH and concentration of the background electrolyte, chiral selector concentration, applied voltage, system temperature and injection parameters were studied and optimized in order to obtain increased chiral resolution and shorten analysis time. The results were compared with those obtained with native and derivatized neutral cyclodextrins (β-cyclodextrin and hydroxypropryl-β-cyclodextrin). All model molecules were resolved using Captisol® as chiral additive and optimized electrophoretic conditions, proving the efficiency of this anionic derivative of β- cyclodextrin as chiral selector in capillary electrophoresis.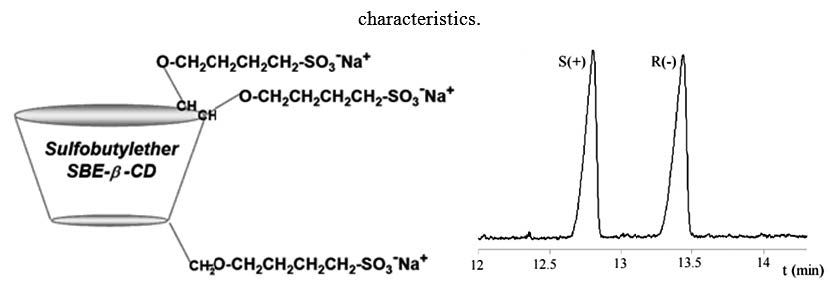
References
- L.A. Nguyen, H. He, C. Pham-Huy, Int. J. Biomed. Sci. 2, 85, (2006).
- G. Gübitz, M.G. Schmid (eds.), Chiral Separations – Methods and Protocols. Humana Press, Totowa, New Jersey, 2004.
- P. Schmitt-Kopplin (eds.), Capillary electrophoresis – Methods and Protocols. Humana Press, Totowa, New Jersey, 2008.
- P. Mikus, Chiral Capillary Electrophoresis in current Pharmaceutical and Biomedical Analysis, InTech, Rijeka, 2012.
- T. Schnitt, H. Engelhardt, Chromatographia 37, 475, (1993).
- G. Gübitz, M.G. Schmid, J. Chromatogr. A 792, 179, (1997).
- G. Gübitz, M.G. Schmid, J. Chromatogr. A 1204, 140, (2008).
- L. Suntornsuk, Anal. Bioanal. Chem. 398, 29, (2010).
- S. Sotthivirat, J.L. Haslam, V.J. Stella, Int. J. Pharm. 330, 73, (2007).
- D.R. Luke, K. Tomaszewki, B. Damle, H.T. Schlamm, J. Pharm. Sci. 99, 3291, (2010).
- A. Espada, M. Molina-Martin, Drug Discov. Today 17, 396, (2012).
- R.J. Tait, D.O. Thompson, V.J. Stella, J.F. Stobaugh, Anal. Chem. 66, 4013, (1994).
- C. Desidorio, S. Fanali, J. Chromatogr. A 716, 183, (1995).
- D.J. Skanchy, G.H. Xie, R.J. Tait, E. Luna, C. Demarest, J.F. Stobaugh, Electrophoresis 20, 2638, (1999).
- X. Ren, Y Dong, J. Liu, A. Huang, H. Liu, Y. Sun, Z. Sun, Chromatographia 50, 363, (1999).
- M. Taschwer, M.G. Hofer, M.G. Schmid, Electrophoresis 35, 2793, (2014).
- J:H. Block, J.M Beale (eds.), Wilson and Gisvold’s textbook of Organic Medicinal and Pharmaceutical Chemistry. 12th edition, Lippincott Williams&Wilkins, Philadelphia, 2011.
- Martindale, The Complete Drug Reference, 37th edition, Pharmaceutical Press, London, 2011.
- M. Dolezalova, S. Fanali, Electrophoresis 21, 3264, (2000).
- G.S. Yang, D.M. Chen, Y. Yang, B. Tang, J.J. Gao, H.Y. Aboul-Enein, B. Koppenhoefer, Chromatographia 62, 441, (2005).


