- Clay,
- Nylon 6,
- 6,
- spherulites,
- Composites
Copyright (c) 2017 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
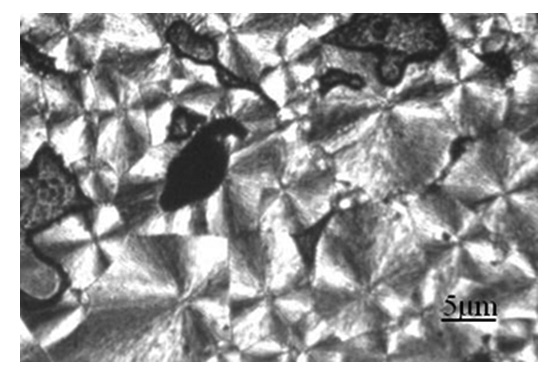
Clay/nylon 6,6 nanocomposites were prepared via solution casting technique. Kaolin clay was used as filler in polymer matrix. The morphological study presented that clay particles were dispersed and embedded well in nylon 6,6 matrix. The thermal stability of the clay/nylon 6,6 nanocomposites (20-50 ºC) were higher than neat nylon 6,6 polymer. The polarized optical microscopy (POM) analyses showed that the spherulites size of clay/nylon 6,6 nanocomposite reduced highly as compare to neat nylon 6,6. The decrease in size of spherulites after loading the clay is might due to nucleation effect of kaolin clay in the nylon 6,6 polymer. The mechanical properties of nanocomposite were decreased as increased kaolin amount. The solvent uptake study presented that the nanocomposite uptake was low as compared to neat nylon 6,6. It was also found that the solvent uptake decreased as increased the amount of kaolin in nylon 6,6.
References
- Giannelis, E. P.Adv. Mater., 8, 29-35 (1996).
- Figiel, L. Computational Mater. Sci., 84, 244–254 (2014).
- LeBaron, P. C.; Wang, Z.; Pinnavaia, T. J. Appl. Clay. Sci., 15, 11-29 (1999).
- Vaia, R. A.; Price, G.; Ruth, P. N.; Nguyen, H. T.; Lichtenhan, J. Appl. Clay. Sci., 15, 67-92 (1999).
- Biswas, M.; Ray, S. S. Adv. Polym. Sci., 155, 167-221 (2001).
- Giannelis, E. P.; Appl. Organomet. Chem., 12, 675-680 (1998).
- Xu, R.; Manias, E.; Snyder, A. J.; Runt, J. Macromolecules, 34, 337-339 (2001).
- Madakbas, S.; Akmakc, E.; Kahraman, M.V.; Esmer, K. Chemical Papers, 67, 1048–1053 2013.
- Agag, T.; Takeichi, T. Polymer, 41, 7083-7090 (2000).
- Usuki, A.; Kawasumi, M.; Kojima, Y.; Okada, A.; Kurauchi, T.; Kamigaito, O. J. Mater. Res. 8, 1179-1184 (1993).
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T. Mater. Res., 8, 1185-1189 (1993).
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Kurauchi, T.; Kamigaito, O. J. Appl. Polym. Sci., 49, 1259-1264 (1993).
- Gilman, J. W.; Kashiwagi, T.; Lichtenhan, J. D. SAMPE. J., 33, 40-46 (1997).
- Okada, A.; Usuki, A. Mater. Sci. Eng. C., 3, 109-115 (1995).
- Sepulveda, M. J.; Vallyathan, V.; Attfield, M. D.; Piacitelli, L.; Tucker, J. H. Am. Rev. Respir. Dis., 127, 231-235 (1983).
- Grim, R. E. Clay mineralogy, 2nd Edd. New York, McGraw-Hill, 1968, p. 596.
- Orellana, F.; Lisperguer, J.; Nuñez, C. J. Chil. Chem. Soc., 59, 2389-2393 (2014).
- Wan, T; Du, T.; Wang, B.; Zeng, W.; Clifford, M. Polym. Compos., 2012, 33, 2271-2276.
- Rathi, S.; dahiya, J. B. Indian J. Chem., 51A, 1677-1685(2012).
- Venkataramani, S.; Lee, J. H.; Park, M. G.; Kim, S. C. J. Macromol. Sci. Part A. Pure Appl. Chem., 46, 65-73 (2009).
- Mansoori, Y.; Sanaei, S. S.; Zamanloo, M.-R.; Imanzadeh, G.; Atghia, S. V. Bull. Mater. Sci., 36, 789-798 (2013).
- Bellotto, M.; gualtieri, A.; Artioli, G.; clark, S. M. Phys. Chem. Minerals., 22, 207-217 (1995).
- Saeed, K.; Khan, I. Iran. Polym. J., 23, 53–58 (2014).


