FIRST REPORT ON THE HPLC PROFILE, IN VITRO AND IN SILICO BIOACTIVITIES OF Arbutus unedo L. FRUIT ETHANOLIC EXTRACT FROM ALGERIA.

- Arbutus unedo,
- HPLC analysis,
- toxicity prediction,
- antioxidan,
- anti-inflammatory
- in silico antimicrobial ...More
Copyright (c) 2025 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
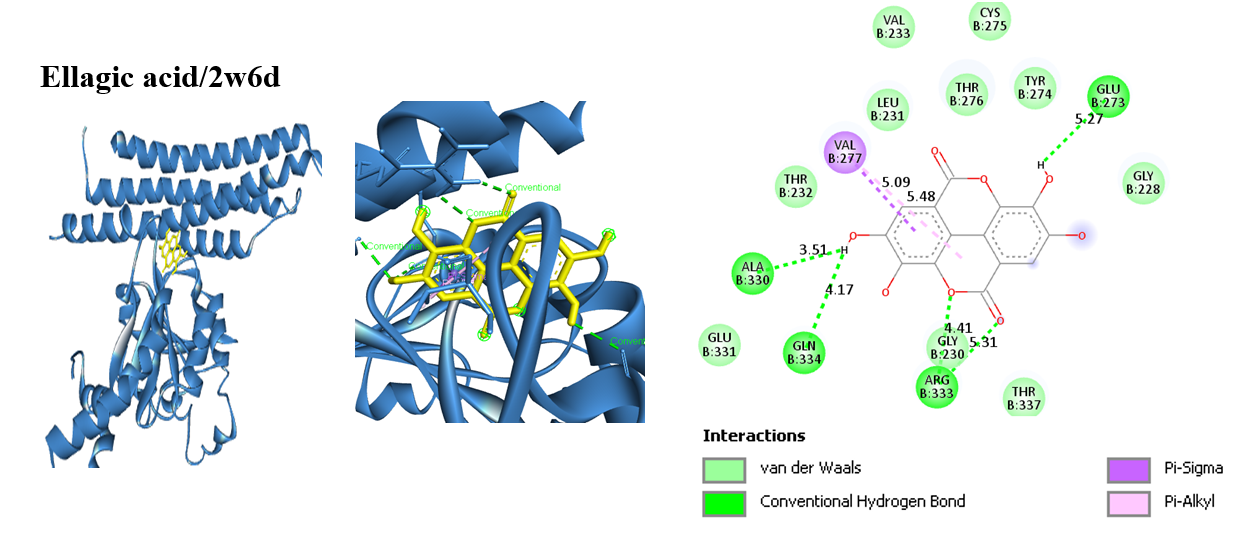
Arbutus unedo L., commonly known as the Strawberry tree, is gaining increasing interest due to its traditional, industrial, and medicinal applications. This study evaluates the in vitro and in silico biological activities of the ethanolic extract of A. unedo fruit, namely its antioxidant, anti-inflammatory and antibacterial properties. HPLC analysis was carried out for the determination of the main components of the extract. Antioxidant activity was assessed via DPPH radical scavenging method, ABTS, metal chelation and β-carotene/linoleic acid bleaching assays while the antiinflammatory activity was done via the inhibition of albumin denaturation method. The in vitro antibacterial activity was evaluated by the disk diffusion method against four ATTC strains. Molecular docking was performed using Autodock Vina PyRx docking techniques against ten bacterial protein targets. HPLC analysis identified 11 compounds whose majority components are: chlorogenic acid (22.66µg/mL) and gallic acid (15.43µg/mL). The extract exhibited strong antioxidant potential, with IC50 values of 0.1 ± 0.007 mg/mL for DPPH, 0.021 ± 0.02 mg/mL for ABTS, and 0.011 ± 0.006 mg/mL for iron chelation. The β-carotene/linoleic acid test showed inhibition rates ranging from 35.43% ± 0.03 to 89.42% ± 0.05. Additionally, the in vitro anti-inflammatory activity revealed an inhibitory effect of 92.97% compared to aspirin (97.40%) at 20 µg/mL. Naringenin, ellagic acid and chlorogenic acid are the best antibacterial candidates with binding energies of less than -8 kcal/mol and more bacterial targets bound. Ferulic acid, methyl gallate, caffeic acid, synergistic acid, and coumaric acid are the safest and pharmacokinetically favorable, while gallic acid, naringenin, and chlorogenic acid have limitations as toxicity or poor absorption. These findings support the traditional medicinal use of A. unedo and highlight its potential as a natural bioactive source.
References
- X. Santiso, L. Lopez, R. Retuerto, R. Barreiro, Population Structure of a Widespread Species under Balancing Selection: The Case of Arbutus unedo L., Front. Plant Sci. 6 (2016). https://doi.org/10.3389/fpls.2015.01264.
- S. Morgado, M. Morgado, A. Plácido, F. Roque, A. Duarte, Arbutus unedo L.: From Traditional Medicine to Potential Uses in Modern Pharmacotherapy, Journal of Ethnopharmacology 225 (2018). https://doi.org/10.1016/j.jep.2018.07.004.
- Ž. Maleš, D. Šarić, M. Bojić, Quantitative Determination of Flavonoids and Chlorogenic Acid in the Leaves of Arbutus unedo L. Using Thin Layer Chromatography, Journal of Analytical Methods in Chemistry 2013 (2013) 1–4. https://doi.org/10.1155/2013/385473.
- Ali Ghasemzadeh, Flavonoids and phenolic acids: Role and biochemical activity in plants and human, J. Med. Plants Res. 5 (2011). https://doi.org/10.5897/JMPR11.1404.
- T. Hussain, B. Tan, Y. Yin, F. Blachier, M.C.B. Tossou, N. Rahu, Oxidative Stress and Inflammation: What Polyphenols Can Do for Us?, Oxid Med Cell Longev 2016 (2016) 7432797. https://doi.org/10.1155/2016/7432797.
- E. Žerovnik, S. Ventura, N.K. Jerala, Special Issue: “Inflammation, Oxidative Stress and Protein Aggregation; Any Links?,” Cells 9 (2020) 2461. https://doi.org/10.3390/cells9112461.
- T. Akaike, Host defense and oxidative stress signaling in bacterial infection, Japanese Journal of Bacteriology 70 (2015) 339–349. https://doi.org/10.3412/jsb.70.339.
- P.N. Chuah, D. Nyanasegaram, K.-X. Yu, R. Mohamed Razik, S. Al-Dhalli, C.S. Kue, K. Shaari, C.H. Ng, Comparative conventional extraction methods of ethanolic extracts of Clinacanthus nutans leaves on antioxidant activity and toxicity, BFJ 122 (2020) 3139–3149. https://doi.org/10.1108/BFJ-02-2020-0085.
- W. Nouioua, G. Sofiane, Antioxidant, anti-inflammatory and antimicrobial activities of aqueous and methanolic extract of Rosmarinus eriocalyx Jord. & Fourr., Int. J. Bio. Chem. Sci 14 (2020) 254–262. https://doi.org/10.4314/ijbcs.v14i1.21.
- A. Dapkevicius, R. Venskutonis, T.A. van Beek, J.P.H. Linssen, Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania, Journal of the Science of Food and Agriculture 77 (1998) 140–146. https://doi.org/10.1002/(SICI)1097-0010(199805)77:1<140::AID-JSFA18>3.0.CO;2-K.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Antioxidant activity applying an improved ABTS radical cation decolorization assay, Free Radical Biology and Medicine 26 (1999) 1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3.
- E.A. Decker, B. Welch, Role of ferritin as a lipid oxidation catalyst in muscle food, J. Agric. Food Chem. 38 (1990) 674–677. https://doi.org/10.1021/jf00093a019.
- Z. Akkouche, L. Aissat, K. Madani, Z. Akkouche, L. Aissat, K. Madani, Effect of Heat on Egg White Proteins, in: International Conference on Applied Life Sciences, IntechOpen, 2012. https://doi.org/10.5772/intechopen.84112.
- EUCAST, Antimicrobial susceptibility testing The European Committee on Antimicrobial Susceptibility Testing, (2022). https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2022_manuals/Manual_v_10.0_EUCAST_Disk_Test_2022.pdf.
- C.A. Lipinski, Lead- and drug-like compounds: the rule-of-five revolution, Drug Discovery Today: Technologies 1 (2004) 337–341. https://doi.org/10.1016/j.ddtec.2004.11.007.
- L. Adjissi, N. Chafai, K. Benbouguerra, I. Kirouani, A. Hellal, H. Layaida, M. Elkolli, C. Bensouici, S. Chafaa, Synthesis, characterization, DFT, antioxidant, antibacterial, pharmacokinetics and inhibition of SARS-CoV-2 main protease of some heterocyclic hydrazones, Journal of Molecular Structure 1270 (2022) 134005. https://doi.org/10.1016/j.molstruc.2022.134005.
- R. Jiamjariyatam, P. Phucharoenrak, S. Samosorn, K. Dolsophon, W. Lorliam, S. Krajangsang, P. Tantayotai, Influence of Different Extraction Methods on the Changes in Bioactive Compound Composition and Antioxidant Properties of Solid-State Fermented Coffee Husk Extracts, The Scientific World Journal 2023 (2023) 1–8. https://doi.org/10.1155/2023/6698056.
- B. Ali, Z. Abderrahim, M. Hassane, S. Marianne, F. Marie-Laure, L. Abdelkhaleq, A. Mohammed, B. Mohamed, Chemical Composition of Cactus Pear Seed Oil: phenolics identification and antioxidant activity, J Pharmacopuncture 25 (2022) 121–129. https://doi.org/10.3831/KPI.2022.25.2.121.
- S. Morgado, M. Morgado, A.I. Plácido, F. Roque, A.P. Duarte, Arbutus unedo L.: From traditional medicine to potential uses in modern pharmacotherapy, Journal of Ethnopharmacology 225 (2018) 90–102. https://doi.org/10.1016/j.jep.2018.07.004.
- M.N.C. Pinheiro, F. Gomes, G. Botelho, I. Rodrigues, R. Mariychuk, L. Symochko, Exploring the Multifaceted Aspects of Strawberry Tree (Arbutus unedo L.) Forests in Portugal, Land 14 (2025) 468. https://doi.org/10.3390/land14030468.
- R. Preti, A.M. Tarola, Influence of geographic origin on the profile and level of phenolic compounds in Italian strawberry tree (Arbutus unedo L.) honey, A. M. 61 (2022).
- R. Di Lorenzo, M.G. Ferraro, C. Carrera, F. Iazzetti, N. Chinchilla, M. Maisto, M.J. Aliaño-González, V. Piccolo, A. Romano, L. Ricci, B. Medronho, A. Marzocchi, M. Piccolo, G.C. Tenore, C. Irace, S. Laneri, Valorization of Arbutus unedo L. Pomace: Exploring the Recovery of Bioactive Phenolic Compounds from Distillation By-Products, Antioxidants 14 (2025) 278. https://doi.org/10.3390/antiox14030278.
- C. Tananaki, D. Kanelis, V. Liolios, M.A. Rodopoulou, F. Papadopoulou, Chemical and Functional Characteristics of Strawberry Tree (Arbutus unedo L.) Honey from Western Greece, Foods 14 (2025) 1473. https://doi.org/10.3390/foods14091473.
- O. Anjos, C.A.L. Antunes, S. Oliveira-Alves, S. Canas, I. Caldeira, Characterisation of Low Molecular Weight Compounds of Strawberry Tree (Arbutus unedo L.) Fruit Spirit Aged with Oak Wood, Fermentation 10 (2024) 253. https://doi.org/10.3390/fermentation10050253.
- H. Zitouni, L. Hssaini, R. Ouaabou, M. Viuda-Martos, F. Hernández, S. Ercisli, S. Ennahli, Z. Messaoudi, H. Hanine, Exploring Antioxidant Activity, Organic Acid, and Phenolic Composition in Strawberry Tree Fruits (Arbutus unedo L.) Growing in Morocco, Plants 9 (2020) 1677. https://doi.org/10.3390/plants9121677.
- I. Moualek, G. Iratni Aiche, N. Mestar Guechaoui, S. Lahcene, K. Houali, Antioxidant and anti-inflammatory activities of Arbutus unedo aqueous extract, Asian Pacific Journal of Tropical Biomedicine 6 (2016) 937–944. https://doi.org/10.1016/j.apjtb.2016.09.002.
- L. Aksoy, E. Kolay, Y. Ağılönü, Z. Aslan, M. Kargıoğlu, Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica, Saudi Journal of Biological Sciences 20 (2013) 235–239. https://doi.org/10.1016/j.sjbs.2013.02.003.
- M. Miao, L. Xiang, Pharmacological action and potential targets of chlorogenic acid, in: Advances in Pharmacology, Elsevier, 2020: pp. 71–88. https://doi.org/10.1016/bs.apha.2019.12.002.
- I. Essaidi, M. Chouaibi, H. Haj Koubaier, S. Bouacida, A. Snoussi, Y. Abassi, N. Bouzouita, Arbutus unedo fruit syrup as a fortifying agent: effect on physicochemical, microbiological, rheological, sensory and antioxidant properties of yoghurt, J Food Sci Technol 60 (2023) 2835–2845. https://doi.org/10.1007/s13197-023-05801-4.
- I. Oliveira, P. Baptista, R. Malheiro, S. Casal, A. Bento, J.A. Pereira, Influence of strawberry tree (Arbutus unedo L.) fruit ripening stage on chemical composition and antioxidant activity, Food Research International 44 (2011) 1401–1407. https://doi.org/10.1016/j.foodres.2011.02.009.
- A.T. Coimbra, Â.F.S. Luís, M.T. Batista, S.M.P. Ferreira, A.P.C. Duarte, Phytochemical Characterization, Bioactivities Evaluation and Synergistic Effect of Arbutus unedo and Crataegus monogyna Extracts with Amphotericin B, Curr Microbiol 77 (2020) 2143–2154. https://doi.org/10.1007/s00284-020-02125-w.
- J.-K. Moon, T. Shibamoto, Antioxidant Assays for Plant and Food Components, J. Agric. Food Chem. 57 (2009) 1655–1666. https://doi.org/10.1021/jf803537k.
- F. Lehfa, H. Belkhodja, F. Sahnouni, Phytochemical Screening Antioxidant and Anti-inflammatory Activities of Polyphenolic Extracts of Strawberry-tree Fruits (Arbutus unedo L), J Apple Biotechnol Rep 10 (2023). https://doi.org/10.30491/jabr.2023.380993.1596.
- S.V.E. Prigent, H. Gruppen, A.J.W.G. Visser, G.A. Van Koningsveld, G.A.H. De Jong, A.G.J. Voragen, Effects of Non-covalent Interactions with 5- O -Caffeoylquinic Acid (Chlorogenic Acid) on the Heat Denaturation and Solubility of Globular Proteins, J. Agric. Food Chem. 51 (2003) 5088–5095. https://doi.org/10.1021/jf021229w.
- A. Precupas, A.R. Leonties, A. Neacsu, R. Sandu, V.T. Popa, Gallic acid influence on bovine serum albumin thermal stability, New J. Chem. 43 (2019) 3891–3898. https://doi.org/10.1039/C9NJ00115H.
- L. Doudach, H.N. Mrabti, S.H. Al-Mijalli, M.R. Kachmar, K. Benrahou, H. Assaggaf, A. Qasem, E.M. Abdallah, B.S. Rajab, K. Harraqui, M. Mekkaoui, A. Bouyahya, M.E.A. Faouzi, Phytochemical, Antidiabetic, Antioxidant, Antibacterial, Acute and Sub-Chronic Toxicity of Moroccan Arbutus unedo Leaves, J Pharmacopuncture 26 (2023) 27–37. https://doi.org/10.3831/KPI.2023.26.1.27.
- S. Ferreira, J. Santos, A. Duarte, A.P. Duarte, J.A. Queiroz, F.C. Domingues, Screening of antimicrobial activity of Cistus ladanifer and Arbutus unedo extracts, Natural Product Research 26 (2012) 1558–1560. https://doi.org/10.1080/14786419.2011.569504.
- A. Ali, G.J. Mir, A. Ayaz, I. Maqbool, S.B. Ahmad, S. Mushtaq, A. Khan, T.M. Mir, M.U. Rehman, In silico analysis and molecular docking studies of natural compounds of Withania somnifera against bovine NLRP9, J Mol Model 29 (2023) 171. https://doi.org/10.1007/s00894-023-05570-z.
- T.P.T. Cushnie, A.J. Lamb, Antimicrobial activity of flavonoids, International Journal of Antimicrobial Agents 26 (2005) 343–356. https://doi.org/10.1016/j.ijantimicag.2005.09.002.
- M. Abdella, C. Lahiri, I. Abdullah, A. Anwar, Antibacterial Evaluation of Gallic Acid and its Derivatives against a Panelof Multi-drug Resistant Bacteria, MC 20 (2024) 130–139. https://doi.org/10.2174/1573406419666230823104300.
- D. Cota, D. Patil, Antibacterial potential of ellagic acid and gallic acid against IBD bacterial isolates and cytotoxicity against colorectal cancer, Natural Product Research 37 (2023) 1998–2002. https://doi.org/10.1080/14786419.2022.2111560.
- W. Zhang, Q.-M. Zeng, R.-C. Tang, Gallic acid functionalized polylysine for endowing cotton fiber with antibacterial, antioxidant, and drug delivery properties, International Journal of Biological Macromolecules 216 (2022) 65–74. https://doi.org/10.1016/j.ijbiomac.2022.06.186.
- K. Chen, C. Peng, F. Chi, C. Yu, Q. Yang, Z. Li, Antibacterial and Antibiofilm Activities of Chlorogenic Acid Against Yersinia enterocolitica, Front. Microbiol. 13 (2022) 885092. https://doi.org/10.3389/fmicb.2022.885092.
- Y.-J. Le, L.-Y. He, S. Li, C.-J. Xiong, C.-H. Lu, X.-Y. Yang, Chlorogenic acid exerts antibacterial effects by affecting lipid metabolism and scavenging ROS in Streptococcus pyogenes, FEMS Microbiology Letters 369 (2022) fnac061. https://doi.org/10.1093/femsle/fnac061.
- M. Su, F. Liu, Z. Luo, H. Wu, X. Zhang, D. Wang, Y. Zhu, Z. Sun, W. Xu, Y. Miao, The Antibacterial Activity and Mechanism of Chlorogenic Acid Against Foodborne Pathogen Pseudomonas aeruginosa, Foodborne Pathogens and Disease 16 (2019) 823–830. https://doi.org/10.1089/fpd.2019.2678.
- D. Morales, Use of Strawberry Tree (Arbutus unedo) as a Source of Functional Fractions with Biological Activities, Foods 11 (2022) 3838. https://doi.org/10.3390/foods11233838.

