
- sulfoxide compounds,
- (poly)phenols,
- alliin,
- COX-2 inhibition,
- mass spectrometry
- antioxidants ...More
Copyright (c) 2025 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
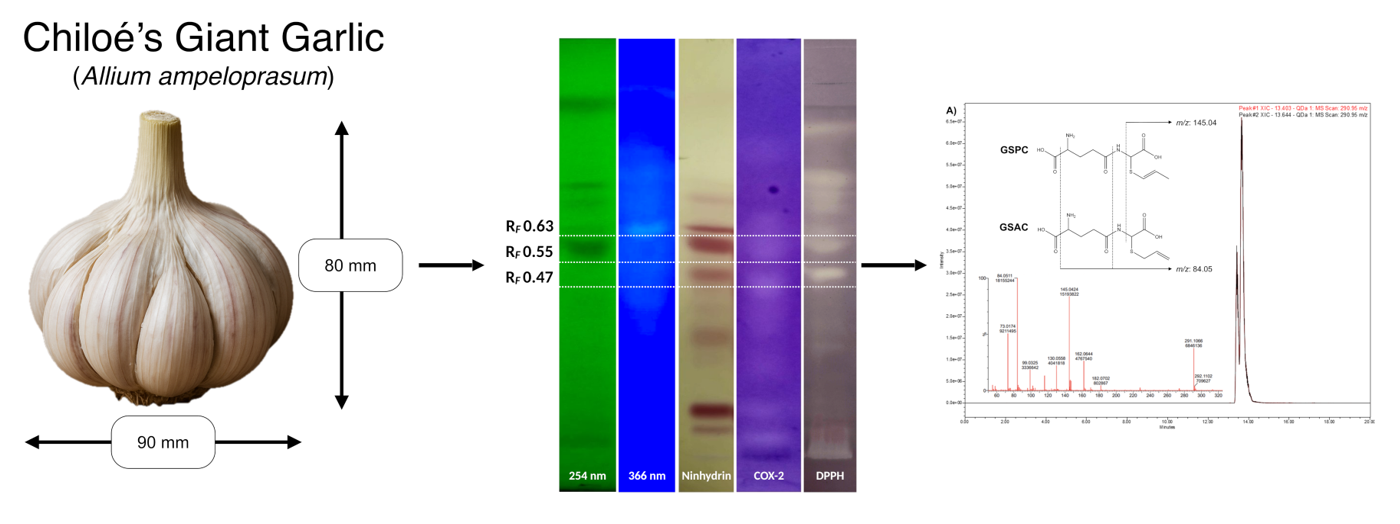
The present work reports the chemical and functional profile of Chiloe’s giant garlic. Applying AOAC's official methods the proximal content of giant garlic was determined finding a moisture content of 62.34±0.35%, carbohydrates 20.64±0.01%, protein 2.80±0.10%, fat (crude) 0.09±0.00%, ash 0.74±0.02%, and fiber (crude) 13.04±0.01%. Three saccharides [sucrose (5.92±0.02 mg g-1), glucose (0.11±0.00 mg g-1) and fructose (0.46±0.01 mg g-1)], four fatty acids [linoleic acid (57.67±0.00%), palmitic acid (23.46±0.00%), oleic acid (7.20±0.00%), and ⍺-linolenic acid (5.06±0.00%)], and three sulfoxide compounds [alliin (2.66±0.90 mg g-1), methiin (9.61±0.33 mg g-1) and isoalliin (5.02±1.24 mg g-1)] were determined by high-performance thin-layer chromatography (HPTLC), gas chromatography (GC) and HPTLC/mass spectrometry (MS), respectively. Functional profile characterization showed an Oxygen Radical Absorbance Capacity (ORAC) value of 0.250±0.001 mmol TE per 100 g-1 and a total (poly)phenols content (TPC) of 40.66±2.08 mg EAG 100 g-1. (Poly)phenols profile analyzed by liquid chromatography (LC)/MS showed only the presence of caffeic acid (0.57±0.05 μg g-1) and rutin (at traces level). Bioactive molecules with antioxidant (DPPH) and COX-2 inhibition activities were identified through HPTLC (bio)autography and MS analysis, finding the presence of tryptophan (antioxidant) and γ-glutamyl-S-allyl-L-cysteine (GSAC), γ-glutamyl-S-(trans-1-propenyl)-L- cysteine (GSPC), alliin and isoalliin with antioxidant and COX-2 inhibitory activity.
References
- Naznin MT, Akagawa M, Okukawa K, Maeda T, Morita N (2008), Characterization of E- and Z-ajoene obtained from different varieties of garlics, Food Chem. 106(3) 1113-1119. http://dx.doi.org/10.1016/j.foodchem.2007.07.041.
- Cardelle-Cobas A, Soria AC, Corzo N, Villamiel M, A comprehensive survey of garlic functionality, in: M. Pacurar, G. Krejci (Eds.), Garlic Consumption and Health Nova Science Publichers, Inc2010, pp. 1-60.
- Liu L, Yeh Y-Y (2000), Inhibition of cholesterol biosynthesis by organosulfur compounds derived from garlic, Lipids 35(2) 197-203. https://doi.org/10.1007/BF02664770.
- Liu L, Yeh Y-Y (2002), S-alk (en) yl cysteines of garlic inhibit cholesterol synthesis by deactivating HMG-CoA reductase in cultured rat hepatocytes, J. Nutr. 132(6) 1129-1134. https://doi.org/10.1093/jn/132.6.1129.
- Charron CS, Milner JA, Novotny JA, Garlic, in: B. Caballero, P.M. Finglas, F. Toldrá (Eds.), Encyclopedia of Food and Health, Academic Press, Oxford, 2016, pp. 184-190. http://dx.doi.org/10.1016/B978-0-12-384947-2.00346-9.
- Khar A, Banerjee K, Jadhav MR, Lawande KE (2011), Evaluation of garlic ecotypes for allicin and other allyl thiosulphinates, Food Chem. 128(4) 988-996. http://doi.org/10.1016/j.foodchem.2011.04.004.
- Peterssen-Fonseca D, Henríquez-Aedo K, Carrasco-Sandoval J, Cañumir-Veas J, Herrero M, Aranda M (2021), Chemometric optimisation of pressurised liquid extraction for the determination of alliin and S-allyl-cysteine in giant garlic (Allium ampeloprasum L.) by liquid chromatography tandem mass spectrometry, Phytochem. Anal 32(6) 1051-1058. https://doi.org/10.1002/pca.3046.
- Latimer GW, Official methods of analysis of AOAC International, AOAC International, Gaithersburg, Md., 2012.
- Watts BM (1964), Food composition and analysis (Triebold, Howard O.; Aurand, Leonard W.), J. Chem. Educ. 41(7) A534. http://doi.org/10.1021/ed041pA534.2.
- Repo-Carrasco-Valencia R, Hellström JK, Pihlava J-M, Mattila PH (2010), Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus), Food Chem. 120(1) 128-133. https://doi.org/10.1016/j.foodchem.2009.09.087.
- Aranda M, Vega MH, Villegas RF (2005), Routine method for quantification of starch by planar chromatography (HPTLC), JPC J. Planar Chromatogr. - Mod. TLC 18(104) 285-289. https://doi.org/10.1556/jpc.18.2005.4.6.
- Vega MH, Jara ET, Aranda MB (2006), Monitoring the dose of florfenicol in medicated salmon feed by planar chromatography (HPTLC), JPC J. Planar Chromatogr. - Mod. TLC 19(109) 204-207. https://doi.org/10.1556/jpc.19.2006.3.6
- Bligh EG, Dyer WJ (1959), A rapid method of total lipid extraction and purification Can. J. Biochem. Physiol. 37(8) 911-917. https://doi.org/10.1139/o59-099.
- IUPAC, Standard Method 2.301,Preparation of Fatty Acid Methyl Ester, in Standard Methods for Analysis of Oils, Fats and Derivatives. 7th Edition, Blackwell, Oxford, 1998.
- Aranda M, Mendoza N, Villegas R (2006), Lipid damage during frozen storage of whole jack mackerel (Trachurus symmetricus murphyi), J. Food Lipids 13(2) 155-166. https://doi.org/10.1111/j.1745-4522.2006.00041.x.
- Carrasco-Sandoval J, Falcó I, Sánchez G, Fabra MJ, López-Rubio A, Rodriguez A, Henríquez-Aedo K, Aranda M (2022), Multivariable optimization of ultrasound-assisted extraction for the determination of phenolic and antioxidants compounds from arrayan (Luma apiculata (DC.) Burret) leaves by microplate-based methods and mass spectrometry, J. Appl. Res. Med. Aromat. Plants 28 100356. https://doi.org/10.1016/j.jarmap.2021.100356.
- Carrasco-Sandoval J, Rebolledo P, Peterssen-Fonseca D, Fischer S, Wilckens R, Aranda M, Henríquez-Aedo K (2021), A fast and selective method to determine phenolic compounds in quinoa (Chenopodium quinoa Will) seeds applying ultrasound-assisted extraction and high-performance liquid chromatography, Chem. Pap. 75(1) 431-438. https://doi.org/10.1007/s11696-020-01313-z.
- Aravena-Sanhueza F, Pérez-Rivera M, Castillo-Felices R, Mundaca-Uribe R, Aranda Bustos M, Peña Farfal C (2020), Determination of antioxidant capacity (ORAC) of greigia sphacelata and correlation with voltammetric methods, J. Chil. Chem. Soc. 65 4925-4928. http://dx.doi.org/10.4067/s0717-97072020000204925
- CAMAG (2008), HPTLC Identification of Garlic (Allium sativum) F-06A, Alliin, APPLICATION NOTES.
- Lopez K, Espinoza-Bello A, Carrasco J, Peña-Farfal C, Aranda M, Henriquez-Aedo K (2023), Multivariate optimization of microwave-assisted enzyme digestion of α-casein for generation of bioactive peptides, J. Chil. Chem. Soc. 68 5963-5968. http://dx.doi.org/10.4067/s0717-97072023000305963.
- Oyarzún P, Carrasco J, Peterssen D, Tereucan G, Aranda M, Henríquez-Aedo K (2023), A high throughput method for detection of cyclooxygenase-2 enzyme inhibitors by effect-directed analysis applying high performance thin layer chromatography-bioassay-mass spectrometry, J. Chromatogr. A 1711 464426. https://doi.org/10.1016/j.chroma.2023.464426.
- Galarce-Bustos O, Pavon J, Henriquez-Aedo K, Aranda M (2019), Detection and identification of acetylcholinesterase inhibitors in Annona cherimola Mill. by effect-directed analysis using thin-layer chromatography-bioassay-mass spectrometry, Phytochem. Anal 30(6) 679-686. https://doi.org/10.1002/pca.2843.
- Galarce-Bustos O, Pavon-Perez J, Henriquez-Aedo K, Aranda M (2019), An improved method for a fast screening of alpha-glucosidase inhibitors in cherimoya fruit (Annona cherimola Mill.) applying effect-directed analysis via high-performance thin-layer chromatography-bioassay-mass spectrometry, J. Chromatogr. A 1608. https://doi.org/10.1016/j.chroma.2019.460415.
- Liu P, Weng R, Sheng X, Wang X, Zhang W, Qian Y, Qiu J (2020), Profiling of organosulfur compounds and amino acids in garlic from different regions of China, Food Chem. 305 125499. https://doi.org/10.1016/j.foodchem.2019.125499.
- Odebunmi E, Oluwaniyi O, Bashiru M (2010), Comparative proximate analysis of some food condiments, J. Appl. Sci. Res. 6(3) 272-274.
- García-Herrera P, Morales P, Fernández-Ruiz V, Sánchez-Mata MC, Cámara M, Carvalho AM, Ferreira ICFR, Pardo-de-Santayana M, Molina M, Tardio J (2014), Nutrients, phytochemicals and antioxidant activity in wild populations of Allium ampeloprasum L., a valuable underutilized vegetable, Food Res. Int. 62 272-279. http://dx.doi.org/10.1016/j.foodres.2014.03.004.
- Ceccanti C, Rocchetti G, Lucini L, Giuberti G, Landi M, Biagiotti S, Guidi L (2021), Comparative phytochemical profile of the elephant garlic (Allium ampeloprasum var. holmense) and the common garlic (Allium sativum) from the Val di Chiana area (Tuscany, Italy) before and after in vitro gastrointestinal digestion, Food Chem. 338 128011. https://doi.org/10.1016/j.foodchem.2020.128011.
- Loppi S, Fedeli R, Canali G, Guarnieri M, Biagiotti S, Vannini A (2021), Comparison of the Mineral and Nutraceutical Profiles of Elephant Garlic (Allium ampeloprasum L.) Grown in Organic and Conventional Fields of Valdichiana, a Traditional Cultivation Area of Tuscany, Italy, Biology (Basel) 10(10). http://doi.org/10.3390/biology10101058.
- Zhang Y, Liu X, Ruan J, Zhuang X, Zhang X, Li Z (2020), Phytochemicals of garlic: Promising candidates for cancer therapy, Biomed. Pharmacother. 123 109730. https://doi.org/10.1016/j.biopha.2019.109730.
- Emir C, Coban G, Emir A (2022), Metabolomics profiling, biological activities, and molecular docking studies of elephant garlic (Allium ampeloprasum L.), Process Biochem. 116 49-59. https://doi.org/10.1016/j.procbio.2022.03.002.
- Beato VM, Orgaz F, Mansilla F, Montaño A (2011), Changes in Phenolic Compounds in Garlic (Allium sativum L.) Owing to the Cultivar and Location of Growth, Plant Foods Hum. Nutr. 66(3) 218-223. http://doi.org/10.1007/s11130-011-0236-2.
- Molina-Calle M, de Medina VS, Priego-Capote F, de Castro MDL (2017), Establishing compositional differences between fresh and black garlic by a metabolomics approach based on LC–QTOF MS/MS analysis, J. Food Compost. Anal. 62(Supplement C) 155-163. https://doi.org/10.1016/j.jfca.2017.05.004.
- Kim S, Park S-L, Lee S, Lee S-Y, Ko S, Yoo M (2016), UPLC/ESI-MS/MS analysis of compositional changes for organosulfur compounds in garlic (Allium sativum L.) during fermentation, Food Chem. 211 555-559. https://doi.org/10.1016/j.foodchem.2016.05.102.
- Siddiqui NA, Mothana RA, Alam P (2016), Quantitative determination of alliin in dried garlic cloves and products by high-performance thin-layer chromatography, Trop. J. Pharm. Res 15(8) 1759-1765. http://doi.org/10.4314/tjpr.v15i8.23.
- Krest I, Keusgen M (2002), Biosensoric flow-through method for the determination of cysteine sulfoxides, Anal. Chim. Acta 469(2) 155-164. https://doi.org/10.1016/S0003-2670(02)00665-7.
- Kim S, Kim D-B, Jin W, Park J, Yoon W, Lee Y, Kim S, Lee S, Kim S, Lee O-H, Shin D, Yoo M (2018), Comparative studies of bioactive organosulphur compounds and antioxidant activities in garlic (Allium sativum L.), elephant garlic (Allium ampeloprasum L.) and onion (Allium cepa L.), Nat. Prod. Res. 32(10) 1193-1197. https://doi.org/10.1080/14786419.2017.1323211.
- Ferioli F, Giambanelli E, D'Antuono LF (2022), Non-volatile cysteine sulphoxides and volatile organosulphur compounds in cloves of garlic (Allium sativum L.) and elephant garlic (Allium ampeloprasum L.) local accessions from northern and central Italy, J. Sci. Food Agric. 102(11) 4744-4751. https://doi.org/10.1002/jsfa.11835.
- Fei MLI, Tong LI, Wei LI, De Yang L (2015), Changes in antioxidant capacity, levels of soluble sugar, total polyphenol, organosulfur compound and constituents in garlic clove during storage, Ind. Crops Prod. 69 137-142. http://dx.doi.org/10.1016/j.indcrop.2015.02.021.
- Nuutila AM, Puupponen-Pimiä R, Aarni M, Oksman-Caldentey K-M (2003), Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity, Food Chem. 81(4) 485-493. https://doi.org/10.1016/S0308-8146(02)00476-4.
- Ciric A, Krajnc B, Heath D, Ogrinc N (2020), Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic, Food Chem. Toxicol. 135 110976. https://doi.org/10.1016/j.fct.2019.110976.
- Chen S, Shen X, Cheng S, Li P, Du J, Chang Y, Meng H (2013), Evaluation of garlic cultivars for polyphenolic content and antioxidant properties, PLoS One 8(11) e79730. http://doi.org/10.1371/journal.pone.0079730.
- García-Villalón AL, Amor S, Monge L, Fernández N, Prodanov M, Muñoz M, Inarejos-García AM, Granado M (2016), In vitro studies of an aged black garlic extract enriched in S-allylcysteine and polyphenols with cardioprotective effects, J. Funct. Foods 27 189-200. http://dx.doi.org/10.1016/j.jff.2016.08.062.
- El-Saber Batiha G, Magdy Beshbishy A, G. Wasef L, Elewa YHA, A. Al-Sagan A, Abd El-Hack ME, Taha AE, M. Abd-Elhakim Y, Prasad Devkota H (2020), Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review, Nutrients 12(3) 872. https://doi.org/10.3390/nu12030872.
- Sadeghi M, Miroliaei M, Fateminasab F, Moradi M (2022), Screening cyclooxygenase-2 inhibitors from Allium sativum L. compounds: in silico approach, J. Mol. Model. 28(1) 12. http://doi.org/10.1007/s00894-021-05016-4.
- Xu N, Chen G, Liu H (2017), Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation, Molecules 22(12). http://doi.org/10.3390/molecules22122066.

