A RAPID LIQUID CHROMATOGRAPHIC METHOD WITH EVAPORATIVE LIGHT SCATTERING DETECTION FOR POSTMORTEM GLUCOSE MEASUREMENT IN VITREOUS HUMOR

- Glucose,
- Postmortem,
- Validation,
- Vitreous Humor
Copyright (c) 2025 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
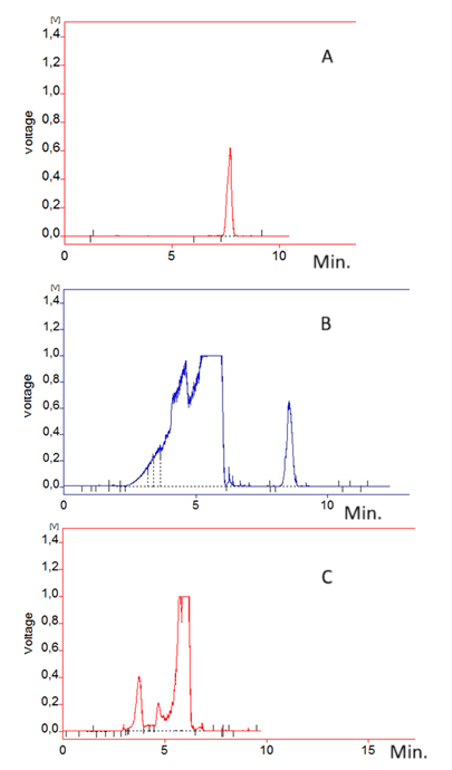
Hyperglycemia is considered a modifiable risk factor that worsens the prognosis of acute myocardial infarction. Accurate glucose measurement is crucial for assessing hyperglycemia in patients who have died from infarction. Since postmortem blood glucose levels are unreliable due to metabolic changes, vitreous humor (VH) provides a reliable alternative for glucose analysis. In this study, a simple and rapid liquid chromatographic method with evaporative light scattering detection (LC-ELSD) was developed and validated for glucose quantification in VH, including a stability study. The method was developed using a Diol column with an isocratic mobile phase consisting of water and acetonitrile (23:77 v/v) at a flow rate of 1 mL/min, achieving a glucose retention time of 8.3 minutes. The column temperature was maintained at 40°C, while the ELSD evaporation temperature was set at 45°C. Validation was performed according to FDA guidelines. Calibration curves demonstrated linearity over a range of 15–300 mg/dL with R² > 0.99. Intraday and interday precision and accuracy were within ±15% of nominal values, except at the LLOQ, where they were within ±20%. The extraction recoveries ranged from 97.82 to 102.66%. The method exhibited high selectivity and specificity, with no interference from endogenous compounds or co-administered drugs. Stability studies confirmed that glucose remained stable in VH for up to 72 hours at -20°C, +4°C, and room temperature. The results demonstrate that the proposed method offers a reliable and efficient approach for glucose determination in VH suitable for forensic applications, eliminating the need for derivatization.
References
- T. I. Khan, M. N. Islam, M. H. Khan, M. Hassan, S. M. Mahmud, F. Naznen. Mymensingh Med. J. 31, 592, (2022).
- S. E. Capes, D. Hunt, K. Malmberg, H. C. Gerstein. Lancet, 355, 773, (2000).
- I. Khan, A. H. Siddiqui, S. Singhal, M. Aslam, A. Perwez, A. Faraz. Diabetes Metab. Syndr. Clin. Res. Rev. 10, S140, (2016)
- J. Chang, G. Zhang, L. Zhang, Y. P. Hou, X. L. Liu, L. Zhang. Cardiovasc. Diabetol. 12, 171, (2013).
- A. Holstein, U. Titze, C. Hess. Exp. Clin. Endocrinol. Diabetes. 128, 239, (2020).
- C. Boulagnon, R. Garnotel, P. Fornes, P. Gillery, (2011). Clin. Chem. Lab. Med. 49, 1265, (2011).
- B. Zilg, K. Alkass, S. Berg, H. Druid. Forensic Sci. Int. 185, 89, (2009).
- N. Baniak, G. Campos- Baniak, A. Mulla, J. Kalra. J. Clin. Exp. Pathol. 5, 1, (2015).
- K. K. Jia, J. Zang. Clin. Chem. Lab. Med. 48, 361, (2010).
- S. Kubihal, A. Goyal, Y. Gupta, R. Khadgawat. Diabetes Metab. Syndr. Clin. Res. Rev 15, 45, (2021).
- S. A. Blana, F. Musshoff, T. Hoeller, R. Fimmers, B. Madea. Forensic Sci. Int. 210, 263, (2011).
- Y. Chen, Q. Yao, L. Zhang, P. Zeng. Front. Chem. 11, 1289211, (2023).
- Y. Chen, Q. Yao, X. Zeng, C. Hao, X. Li, L. Zhang, P. Zeng.. Front. Oncol. 12, 1014159, (2022).
- T. Sato, K. Katayama, T. Arai, T. Sako, H. Tazaki. Res. Vet. Sci. 84, 26, (2008).
- M. Li, P. Li, Y. Ji, Y. Tian, H. Zeng, X. Chen. J. Pharm. Biomed. Anal. 234, 115537, (2023).
- M. Li, D. Yan, M. Hao, X. Huang, Y. Xu, W. Li, W. Liu. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1200, 123277, (2022).
- M. Diepeveen-de Bruin, W. Maho, M. E. C. Buso, N. D. Naomi, E. M. Brouwer-Brolsma, E. J. M. Feskens, M. G. J. Balvers. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1255, 123741, (2023).
- P. Shanmugavelan, S. Y. Kim, J. B. Kim, H. W. Kim, S. M. Cho, S. N. Kim, S. Y. Kim, Y. S. Cho, H. R. Kim. Carbohydr. Res. 380, 112, (2013).
- D. N Lindqvist, H. Æ. Pedersen, L. H. Rasmussen. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1081, 126, (2018).
- S. Sun, H. Wang, J. Xie, Y. Su. Chem. Cent. J. 10, 25, (2016).
- FDA, Food and Drug Administration, Guidance for Industry: Bioanalytical Method Validation, (2018).
- A. Thierauf, F. Musshoff, B. Madea. Forensic Sci. Int. 192, 78, (2009).
- S. Hostiuc, I. Negoi, M. Hostiuc. J. Forensic Leg. Med. 83, 102250 (2021).
- C. Hess, F. Musshoff, B. Madea. Int. J. Legal Med. 125, 163 (2011).

