SYNTHESIS OF NOVEL QUINOLINE DERIVATIVES: A VERSATILE PLATFORM FOR ADVANCED ORGANIC AND MEDICINAL CHEMISTRY

- heterocyclic compounds,
- organic synthesis,
- quinoline
Copyright (c) 2025 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
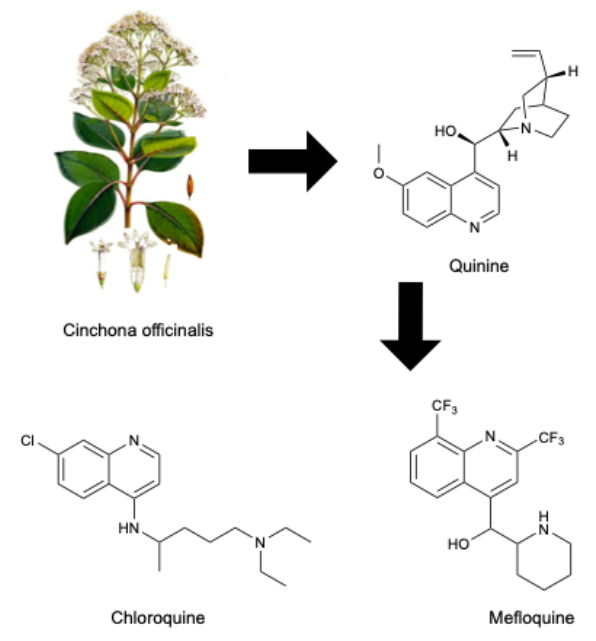
Quinolines are heterocyclic compounds with significant therapeutic potential, initially recognized for their role in treating malaria. Their structural versatility has led to the development of derivatives used in the treatment of various diseases, including Alzheimer's and Parkinson's. Found primarily in plants of the Rutaceae and Rubiaceae families, quinoline derivatives target key enzymes and receptors in the central nervous system. Recent advances focus on enhancing their pharmacokinetic properties to improve efficacy and selectivity in treating neurodegenerative disorders.
References
2. Ajani, O. O., Iyaye, K. T. & Ademosun, O. T. RSC Adv. 12, 18594–18614 (2022).
3. Christensen, S. B. Biomedicines. 9, 472 (2021).
4. Hariyanti, H., Mauludin, R., Sumirtapura, Y. C. & Kurniati, N. F. Biointerface Res Appl Chem. 13, 319 (2022).
5. Gachelin, G., Garner, P., Ferroni, E., Tröhler, U. & Chalmers, I. J R Soc Med. 110, 31–40 (2017).
6. C. S. Pinheiro, L., M. Feitosa, L., O. Gandi, M., F. Silveira, F. & Boechat, N. Molecules. 24, 4095 (2019).
7. Renslo, A. R. ACS Med Chem Lett. 4, 1126–1128 (2013).
8. Belete, T. M. Drug Des Devel Ther. Volume 14, 3875–3889 (2020).
9. Ashley, E. A. & Phyo, A. P. Drugs. 78, 861–879 (2018).
10. Kumar, S., Bhardwaj, T. R., Prasad, D. N. & Singh, R. K. Biomedicine & Pharmacotherapy. 104, 8–27 (2018).
11. Chassagne, F. et al. Front Pharmacol. 11, (2021).
12. Grosu (Dumitrescu), C. et al. Life. 14, 1019 (2024).
13. Wink, M. Medicines. 2, 251–286 (2015).
14. Králová, P. & Soural, M. Eur J Med Chem. 269, 116287 (2024).
15. Thawabteh, A. M. et al. Toxins (Basel). 16, 489 (2024).
16. Li, Z., Chen, K., Rose, P. & Zhu, Y. Z. Front Chem. 10, (2022).
17. Elebiju, O. F., Ajani, O. O., Oduselu, G. O., Ogunnupebi, T. A. & Adebiyi, E. Front Chem. 10, (2023).
18. Shal, B., Ding, W., Ali, H., Kim, Y. S. & Khan, S. Front Pharmacol. 9, (2018).
19. Pavlidis, N. et al. J Inorg Biochem. 217, 111393 (2021).
20. Chen, H. et al. J Enzyme Inhib Med Chem. 38, (2023).
21. Ong, W.-Y., Go, M.-L., Wang, D.-Y., Cheah, I. K.-M. & Halliwell, B. Mol Neurobiol. 58, 106–117 (2021).
22. Zhou, W. et al. Drug Discov Today. 25, 2012–2022 (2020).
23. Martorana, A., La Monica, G. & Lauria, A. Molecules. 25, 4279 (2020).
24. Faheem et al. RSC Adv. 11, 12254–12287 (2021).
25. Szymański, P. et al. Int J Mol Sci. 13, 10067–10090 (2012).
26. Sabandal, P. R., Saldes, E. B. & Han, K.-A. Sci Rep. 12, 20903 (2022).
27. Chen, Z.-R., Huang, J.-B., Yang, S.-L. & Hong, F.-F. Molecules. 27, 1816 (2022).
28. Gajendra, K., Pratap, G. K., Poornima, D. V., Shantaram, M. & Ranjita, G. European Journal of Medicinal Chemistry Reports. 11, 100154 (2024).
29. Hong, S. W., Teesdale-Spittle, P., Page, R. & Truman, P. Neurotoxicology. 93, 163–172 (2022).
30. Waseem, A. M. et al. Results Chem. 13, 101989 (2025).
31. Hernández-Ayala, L. F., Guzmán-López, E. G. & Galano, A. Antioxidants. 12, 1853 (2023).

