HYBRID MATERIAL BASED IN METAL-ORGANIC FRAMEWORK SUPPORTED ON ACTIVATED CARBON AS NOVEL MATERIALS FOR CO2 ADSORPTION FOR ENVIRONMENTAL APPLICATIONS

- Metal-Organic Framework,
- Activated carbon,
- Carbon capture,
- Carbon dioxide
Copyright (c) 2024 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
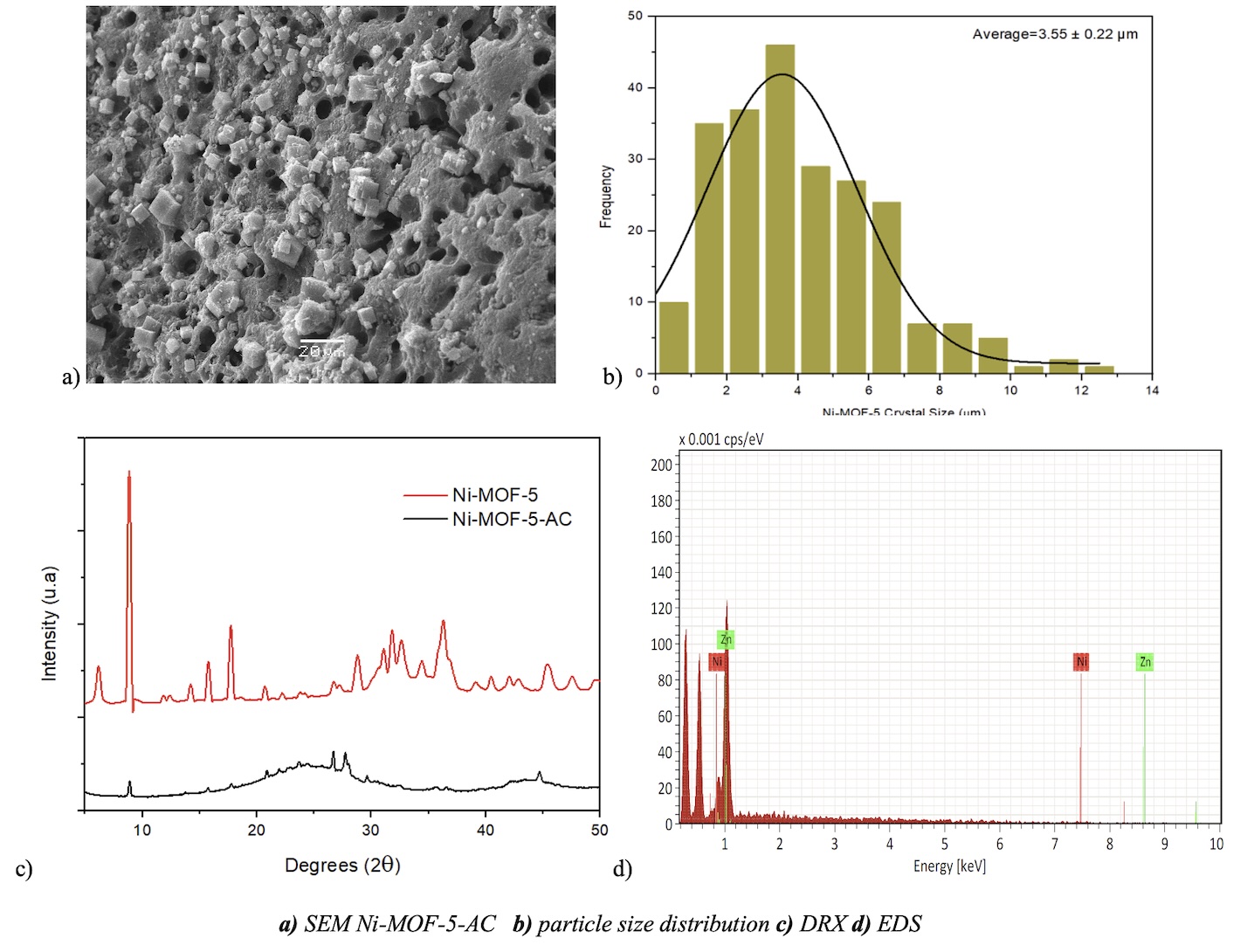
A composite of Ni-MOF-5 (NixZn4-xO(HCOO)3(BCD)3 (0<x<4)) and activated carbon were synthesized through a microwave synthesis method with reflux. Various characterization techniques were performed, including XRD, TGA, SEM, EDS, FT-IR, and BET isotherm. The surface study of the material shows the presence of crystals with a cubic structure of Ni-MOF-5 with sizes smaller than 10 µm. A superficial area of 718 m2/g and thermostability with a mass loss of 4.72% at 459°C of the metal-organic framework were observed. The results for different CO2 concentrations at 1 bar and 25 °C show that the adsorption capacity had a linear relationship between % CO2 and the amount of CO2 adsorbed by the hybrid material. The results also showed that by increasing the working temperature in the CO2 adsorption process and keeping the percentage of CO2 constant, the amount of adsorbed CO2 decreases linearly. The adsorption kinetics of CO2 on the prepared hybrid material are consistent with the intraparticle diffusion model, where diffusion is the rate-limiting step. The adsorption of CO2 is energetically and kinetically favorable due to the micro-mesoporosity of the material that allows the entry of CO2 molecules into the pores.
References
- J. Zhang, J. Shi, S. Tao, L. Wu, and J. Lu, “Cu2O/Ti3C2MXene heterojunction photocatalysts for improved CO2 photocatalytic reduction performance,” Appl. Surf. Sci., vol. 542, no. September 2020, 2021, doi: 10.1016/j.apsusc.2020.148685.
- W. M. S. Mat Latif, N. M. Sabdullah, S. N. Aenun, and N. A. Bosamah, “A review of global carbon capture and storage (CCS) and carbon capture, utilization, and storage (CCUS),” E3S Web Conf., vol. 516, 2024, [Online]. Available: https://doi.org/10.1051/e3sconf/202451601009
- H. Bouaboula, J. Chaouki, Y. Belmabkhout, and A. Zaabout, “Comparative review of Direct air capture technologies: From technical, commercial, economic, and environmental aspects,” Chem. Eng. J., vol. 484, no. January, p. 149411, 2024, doi: 10.1016/j.cej.2024.149411.
- K. Zhang and R. Wang, “A critical review on new and efficient adsorbents for CO2 capture,” Chem. Eng. J., vol. 485, no. January, p. 149495, 2024, doi: 10.1016/j.cej.2024.149495.
- Z. Sun, Y. Liao, X. Zhang, and S. XIangzhen, “Research progress in metal–organic frameworks (MOFs) in CO2 capture from post-combustion coal-fired flue gas: characteristics, preparation, modification and applications,” J. Mater. Chem. A, no. 10, pp. 5174–5211, 2022.
- C. Xiao, J. Tian, Q. Chen, and M. Hong, “Water-stable metal-organic frameworks (MOFs): rational construction and carbon dioxide capture,” Chem. Sci., vol. 15, no. 5, pp. 1570–1610, 2024, doi: 10.1039/d3sc06076d.
- H. K. Okoro, S. O. Ayika, J. C. Ngila, and A. C. Tella, “Rising profile on the use of metal–organic frameworks (MOFs) for the removal of heavy metals from the environment: an overview,” Appl. Water Sci., vol. 8, no. 6, pp. 1–10, 2018, doi: 10.1007/s13201-018-0818-3.
- Ji. Liu, P. Thallapally, B. McGrail, D. Brown, and J. Liu, “Progress in adsorption-based CO2 capture by metal–organic frameworks,” Chem. Soc. Rev., no. 41, pp. 2308–2322, 2012.
- H. Yang et al., “Progress in carbon dioxide separation and capture: A review,” J. Environ. Sci., vol. 20, no. 1, pp. 14–27, 2008, doi: 10.1016/S1001-0742(08)60002-9.
- B. Streppel and M. Hirscher, “BET specific surface area and pore structure of MOFs determined by hydrogen adsorption at 20 K,” Phys. Chem. Chem. Phys., no. 13, pp. 3220–3222, 2011.
- O. K. Farha et al., “De novo synthesis of a metal–organic framework material featuring ultrahigh surface area and gas storage capacities,” Nat. Chem., vol. 2, no. 11, pp. 944–948, 2010, doi: 10.1038/nchem.834.
- J. Letwaba, U. O. Uyor, M. L. Mavhungu, N. O. Achuka, and P. A. Popoola, “A review on MOFs synthesis and effect of their structural characteristics for hydrogen adsorption,” RSC Adv., vol. 14, no. 20, pp. 14233–14253, 2024, doi: 10.1039/D4RA00865K.
- H. Li, M. Eddaoudi, M. O’Keeffe, and O. M. Yaghi, “Design and synthesis of an exceptionally stable and highly porous metal-organic framework,” Nature, vol. 402, no. 6759, pp. 276–279, 1999, doi: 10.1038/46248.
- J. A. Greathouse and M. D. Allendorf, “The Interaction of Water with MOF-5 Simulated by Molecular Dynamics,” J. Am. Chem. Soc., vol. 128, no. 33, pp. 10678–10679, Aug. 2006, doi: 10.1021/ja063506b.
- A. Katoch, R. Bhardwaj, N. Goyal, and S. Gautam, “Synthesis, structural and optical study of Ni-doped Metal-organic framework for adsorption based chemical sensor application,” Vacuum, vol. 158, pp. 249–256, 2018, doi: https://doi.org/10.1016/j.vacuum.2018.09.019.
- J.-M. Yang, Q. Liu, and W.-Y. Sun, “Shape and size control and gas adsorption of Ni(II)-doped MOF-5 nano/microcrystals,” Microporous Mesoporous Mater., vol. 190, pp. 26–31, 2014, doi: https://doi.org/10.1016/j.micromeso.2014.01.020.
- H. Li, W. Shi, K. Zhao, H. Li, Y. Bing, and P. Cheng, “Enhanced Hydrostability in Ni-Doped MOF-5,” Inorg. Chem., vol. 51, no. 17, pp. 9200–9207, Sep. 2012, doi: 10.1021/ic3002898.
- X.-W. Liu, T.-J. Sun, J.-L. Hu, and S.-D. Wang, “Composites of metal–organic frameworks and carbon-based materials: preparations, functionalities and applications,” J. Mater. Chem. A, vol. 4, no. 10, pp. 3584–3616, 2016, doi: 10.1039/C5TA09924B.
- Q. L. Zhu and Q. Xu, “Metal-organic framework composites,” Chem. Soc. Rev., vol. 43, no. 16, pp. 5468–5512, 2014, doi: 10.1039/c3cs60472a.
- K. A. Adegoke et al., “Metal-organic framework composites for electrochemical CO2 reduction reaction,” Sep. Purif. Technol., vol. 341, p. 126532, 2024, doi: https://doi.org/10.1016/j.seppur.2024.126532.
- H. N. Nguyen et al., “Investigation on cost-effective composites for CO2 adsorption from post-gasification residue and metal organic framework,” J. Environ. Sci., vol. 148, pp. 174–187, 2025, doi: https://doi.org/10.1016/j.jes.2023.10.022.
- R. Gonzalez-Olmos, A. Gutierrez-Ortega, J. Sempere, and R. Nomen, “Zeolite versus carbon adsorbents in carbon capture: A comparison from an operational and life cycle perspective,” J. CO2 Util., vol. 55, p. 101791, 2022, doi: https://doi.org/10.1016/j.jcou.2021.101791.
- H. Jensen et al., “Determination of size distributions in nanosized powders by TEM, XRD, and SAXS,” J. Exp. Nanosci., vol. 1, no. 3, pp. 355–373, Sep. 2006, doi: 10.1080/17458080600752482.
- M. Malbenia John et al., “An overview on the key advantages and limitations of batch and dynamic modes of biosorption of metal ions,” Chemosphere, vol. 357, p. 142051, 2024, doi: https://doi.org/10.1016/j.chemosphere.2024.142051.
- H. Zhao, H. Song, and L. Chou, “Nickel nanoparticles supported on MOF-5: Synthesis and catalytic hydrogenation properties,” Inorg. Chem. Commun., vol. 15, pp. 261–265, 2012, doi: https://doi.org/10.1016/j.inoche.2011.10.040.
- T. Birsa Čelič et al., “Study of Hydrothermal Stability and Water Sorption Characteristics of 3-Dimensional Zn-Based Trimesate,” J. Phys. Chem. C, vol. 117, no. 28, pp. 14608–14617, Jul. 2013, doi: 10.1021/jp4036327.
- J. Ling, A. Ntiamoah, P. Xiao, P. A. Webley, and Y. Zhai, “Effects of feed gas concentration, temperature and process parameters on vacuum swing adsorption performance for CO2 capture,” Chem. Eng. J., vol. 265, pp. 47–57, 2015, doi: https://doi.org/10.1016/j.cej.2014.11.121.
- H. Ramezanipour Penchah, A. Ghaemi, and F. Jafari, “Piperazine-modified activated carbon as a novel adsorbent for CO2 capture: modeling and characterization,” Environ. Sci. Pollut. Res., vol. 29, no. 4, pp. 5134–5143, 2022, doi: 10.1007/s11356-021-16040-5.
- C. Guan, S. Liu, C. Li, Y. Wang, and Y. Zhao, “The temperature effect on the methane and CO2 adsorption capacities of Illinois coal,” Fuel, vol. 211, pp. 241–250, 2018, doi: https://doi.org/10.1016/j.fuel.2017.09.046.
- P. Puspitasari, S. Sukarni, and A. Hamzah, “Effect of MnFe2O4 Nanoparticles to Reduce CO and HC Levels on Vehicle Exhaust Gas Emissions,” J. Mech. Eng. Sci. Technol., vol. 2, no. 1, pp. 27–37, 2018, doi: 10.17977/um016v2i12018p027.
- G.-B. Elvira, G.-C. Francisco, S.-M. Víctor, and M.-L. R. Alberto, “MgO-based adsorbents for CO2 adsorption: Influence of structural and textural properties on the CO2 adsorption performance,” J. Environ. Sci., vol. 57, pp. 418–428, 2017, doi: https://doi.org/10.1016/j.jes.2016.11.016.

