PHYTOCHEMICAL ANALYSIS, ANTIOXIDANT PROPERTIES AND PHENOLIC ACIDS DETERMINATION OF DATE PALM (Phoenix dactylifera L.) LEAVES AND ROOTS EXTRACTS

- Phenolic compounds,
- Flavonoids,
- DPPH,
- HPLC-DAD,
- Phoenix dactylifera L
Copyright (c) 2025 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
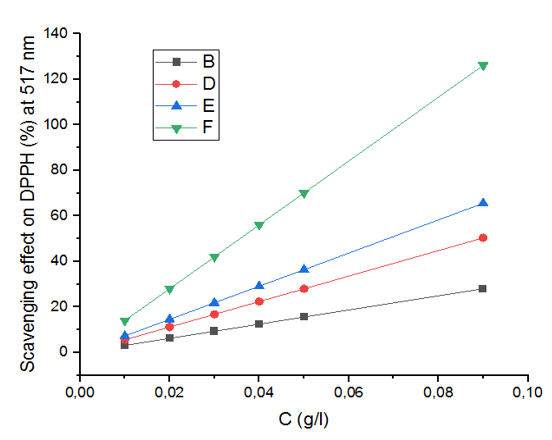
The date palm (Phoenix dactylifera L.) is a vital phytogenetic resource in arid regions. The roots and leaves are traditionally used for various purposes, including medicine. Despite extensive research on date palm fruits, the biochemical properties of its by-products remain underexplored. This study investigates the bioactive compounds in the leaves and roots of date palm cultivars. A reflux apparatus was used to extract total phenolic content (TPC), measured using the Singleton and Rossi method, while total flavonoid content (TFC) was determined using aluminum trichloride. Antioxidant activity was assessed using the DPPH assay, and HPLC-DAD analysis identified and characterized phenolic acids. The results revealed very high TPC levels in both leaves and roots supporting their traditional uses, reaching up to 825.63 ± 0,1 mg GAE/100 g dry weight in TAD leaves. TFC ranged from 18.81±0,15 to 117.85±0,2 mg EQ/100 g dry weight and correlated significantly with TPC. The extracts demonstrated significant antiradical efficacy compared to vitamin C, suggesting that all parts of the date palm are excellent sources of natural antioxidants. Twelve cell wall-bound and soluble phenolic acids were identified, several of which were previously unreported in this plant. Leaves predominantly contained ferulic acid, p-coumaric acid, and sinapic acid, while roots were rich in p-hydroxybenzoic acid. Additionally, several hydroxybenzoic and hydroxycinnamic acids implicated in plant defense mechanisms were identified. These findings highlight the dual importance of conserving local plant biodiversity and exploring date palm phenolics for applications in food, pharmaceuticals, and organic agriculture.Haut du formulaireBas du formulaire
References
- Newman, D. J., & Cragg, G. M. (2016). Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod., 75(3), 311-335.
- Panche, A. N., Diwan, A. D., & Chandra, S. R. (2016). Flavonoids: an overview. J. Nutr. Sci., 5, e47.
- Manach, C., Scalbert, A., Morand, C., Rémésy, C., & Jiménez, L. (2004). Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr., 79(5), 727-747.
- Shahidi, F., & Ambigaipalan, P. (2015). Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods, 18, 820-897.
- Chemat, F., & Vian, M. A. (Eds.). (2019). Phenolic compounds: Natural sources, importance and applications. CRC Press.
- Macheix, J. J., Fleuriet, A., & Billot, J. (Eds.). (2005). Fruit Phenolics. CRC Press.
- Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic., 16, 144–158.
- Chang, C., Yang, M., Wen, H., & Chern, J. (2002). Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal., 10(3), 178-182.
- Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol., 28(1), 25-30.
- González, L. F., Rojas, M. C., & Perez, F. J. (1999). Diferulate and lignin formation is related to biochemical differences of wall-bound peroxidases. Phytochemistry, 50, 711-717.
- Pérez-Jiménez, J., Neveu, V., Vos, F., & Scalbert, A. (2010). Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. Eur. J. Clin. Nutr., 64(Suppl 3), S112-S120.
- Rouiha, Z., Ouahrani, M., & Laouini, S. (2016). Evaluation of Phenolic Content and Antioxidant Capacity of leaf extract from Phoenix Dactylifera L obtained by different pH of aqueous extraction. J. Pharm. Res., 10(1), 1-7.
- John, J. A., & Shahidi, F. (2019). Phenolic content, antioxidant and anti-inflammatory activities of seeds and leaves of date palm (Phoenix dactylifera L.). J. Food Bioactives, 5, 120–130. https://doi.org/10.31665/JFB.2019.5179
- Al Kharusi, L., Al Yahyai, R., & Yaish, M. W. (2019). Antioxidant Response to Salinity in Salt-Tolerant and Salt-Susceptible Cultivars of Date Palm. Agriculture, 9(1), 8. https://doi.org/10.3390/agriculture9010008
- Ziouti, A. C., Modafar, E. L., Fleuriet, A. S., Boustani, E. L., & Macheix, J. J. (1996). Phenolic compounds in Date palm cultivars sensitive and resistant to Fusarium oxysporum. Biol. Plant., 38, 451-457.
- Saleh, E., Tawfik, M., & Abu-Tarboush, H. (2011). Phenolic Contents and Antioxidant Activity of Various Date Palm (Phoenix dactylifera L.) Fruits from Saudi Arabia. Food Nutr. Sci., 2(10), 1134-1141. doi: 10.4236/fns.2011.210152
- Miller, D. D., Li, T., & Liu, R. H. (2014). Antioxidants and Phytochemicals. In R. H. Liu (Ed.), Reference Module in Biomedical Sciences. Elsevier. ISBN 9780128012383. https://doi.org/10.1016/B978-0-12-801238-3.00236-1.
- Abramovič, H. (2015). Antioxidant Properties of Hydroxycinnamic Acid Derivatives: A Focus on Biochemistry, Physicochemical Parameters, Reactive Species, and Biomolecular Interactions. In V. R. Preedy (Ed.), Coffee in Health and Disease Prevention (pp. 843-852). Academic Press.
- Grayer, R. J., & Harborne, J. B. (1994). A Survey of Antifungal Compounds from Higher Plants, 1982-1993. Phytochemistry, 37, 19-42.
- Saaidi, M. (1990). Amélioration génétique du palmier dattier critères de sélection, techniques et résultats. Options Méditerranéennes, 11, 133-154.
- El Modafar, C. (2010). Mechanisms of date palm resistance to bayoud disease: Current state of knowledge and research prospects. Physiol. Mol. Plant Pathol., 74, 287-294.
- Belarbi-Halli, R., & Mangenot, F. (1985). Bayoud disease of date palm: ultrastructure of root infection through pneumatodes. Can. J. Bot., 64, 1703-1711.
- Djerbi, M. (1982). Bayoud disease in North Africa: history, distribution, diagnostics, and control. Date Palm J., 2, 153-198.
- Saaidi, M. (1992). Comportement au champ de 32 cultivars de palmier dattier vis-à-vis du Bayoud: 25 années d’observations. Agronomie, 12, 359-370.
- Saeed, M. K., Zahra, N., Sarwar, A., Ullah, N., Aziz, T., Rahman, S. U., Ihsan, T., Alharbi, M., Alshammari, A., & Alasmari, A. F. (2023). Oxygen reactive species effectively scavenged by various extracts of leaves and bark of Eucalyptus globulus. J. Chilean Chem. Soc., 68(3), 5895.
- Kriaa, W., Fetoui, H., Makni, M., Zeghal, N., & Drira, N. E. (2012). Phenolic Contents and Antioxidant Activities of Date Palm (Phoenix dactylifera L.) Leaves. Int. J. Food Prop., 15(6), 1220–1232. https://doi.org/10.1080/10942912.2010.514673
- Cushnie, T. P., & Lamb, A. J. (2005). Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents, 26(5), 343-356.
- Middleton, E., Kandaswami, C., & Theoharides, T. C. (2000). The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev., 52(4), 673-751.
- Singh, R., Singh, S., Kumar, S., & Arora, S. (2007). Evaluation of antioxidant potential of ethyl acetate extract/fractions of Acacia auriculiformis A. Cunn. Food Chem. Toxicol., 45, 1216–1223.
- Halliwell, B. (2007). Dietary polyphenols: Good, bad, or indifferent for your health? Cardiovasc. Res., 73(2), 341-347.
- Kebede, M., & Admassu, S. (2019). Application of antioxidants in food processing industry: Options to improve the extraction yields and market value of natural products. Adv. Food Technol. Nutr. Sci. Open J., 5(2), 38-49. doi: 10.17140/AFTNSOJ-5-155
- El Hadrami, A., El Idrissi-Tourane, A., El Hassni, M., Daayf, F., & El Hadrami, I. (2005). Toxin-based in-vitro selection and its potential application to date palm for resistance to the bayoud Fusarium wilt. C. R. Biol., 328, 732-744.
- Boulenouar, N., Marouf, A., & Cheriti, A. (2011). Antifungal activity and phytochemical screening of extracts from Phoenix dactylifera L. cultivars. Nat. Prod. Res., 25, 1999-2002.
- Niedzwiecki, A., Roomi, M. W., Kalinovsky, T., Rath, M., & Roomi, N. W. (2007). Modulation of inflammatory C-reactive protein and cytokine secretion by selected flavonoids. In Vivo, 21(2), 399-404.
- González-Gallego, J., García-Mediavilla, M. V., Sánchez-Campos, S., Tuñón, M. J., & Fruitós, M. (2010). Fruit polyphenols, immunity and inflammation. Br. J. Nutr., 104(S3), S15-S27.
- Ziouti, A., El Boustani, E., & Macheix, J. J. (1994). Cell wall-bound phenols in date palm leaves and roots: Identification and histochemical localization. Acta Hortic., 381, 276-279.
- Boucenna-Mouzali, B., Gaceb-Terrak, R., & Rahmania, F. (2018). GC–MS Analysis of Cell Wall-Bound Phenolic Compounds and Lignin Quantification in Date Palm Cultivars that are Resistant or Susceptible to Fusarium oxysporum f. sp. albedinis. Arab. J. Sci. Eng., 43, 63-71.
- Funk, S., & Brodelius, R. E. (1990). Phenylpropanoid metabolism in suspension cultures of Vanilla planifolia Andr.: Effects of precursor feeding and metabolic inhibitors. Plant Physiol., 94, 95-101.
- Ryals, J. A., Neuenschwander, U. H., Willits, M. G., Molina, A., Steiner, H. Y., & Hunt, M. D. (1996). Systemic acquired resistance. Plant Cell, 8, 1809-1819.
- Gaceb-Terrak, R. (2011). Contribution to the knowledge of date palm (Phoenix dactylifera L.) causative agent of bayoud (Fusarium oxysporum f. sp. albedinis) interactions by phytochemical analyzes of lipids and phenylpropanoids). Acta Bot. Gall., 158(2), 285-287. DOI: 10.1080/12538078.2011.10516273
- El Hadrami, I., Ramos, T., El Bellaj, M., & El Idrissi-Tourane, A. (1997). A sinapic derivative as an induced defense compound of date palm against Fusarium oxysporum f. sp. albedinis, the agent causing Bayoud disease. Phytopathol. Z., 145(8-9), 329-333.
- Dihazi, A., Jaiti, F., Zouine, J., Hassni, M. E., & Hadrami, I. E. (2003). Effect of salicylic acid on phenolic compounds related to date palm resistance to Fusarium oxysporum f. sp. albedinis. Phytopathol. Mediterr., 42, 9-16.
- Fry, S. C. (1986). Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu. Rev. Physiol., 26, 165-186.
- Ikegawa, T., Mayama, S., Nakayashiki, H., & Kato, H. (1996). Accumulation of diferulic acid during the hypersensitive response of oat leaves to Puccinia coronata f. sp. avena and its role in the resistance of oat tissues to cell wall degrading enzymes. Physiol. Mol. Plant Pathol., 37, 245-256.
- Matern, U., & Grimmig, B. (1993). Polyphenols in plant pathology. In A. Scalbert (Ed.), Polyphenolic Phenomena (pp. 143-147). INRA Paris.
- El Modafar, C., & El Boustani, E. (2000). Changes in cell wall-bound phenolic compounds and lignin in roots of date palm cultivars differing in susceptibility to Fusarium oxysporum f. sp. albedinis. J. Phytopathol., 148, 405-411.
- El Modafar, C., & El Boustani, E. (2001). Cell wall-bound phenolic acid and lignin contents in date palm as related to its resistance to Fusarium oxysporum. Biol. Plant., 44, 125-130.

