SYNTHESIS, ELECTRONIC AND PHOTOPHYSICAL PROPERTIES OF 3,8-DIAROMATIC-1,10-PHENANTHROLINE MOLECULES

- 1,10-phenantroline,
- photophysical,
- voltammetry,
- 3,8-diaromatic
Copyright (c) 2025 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
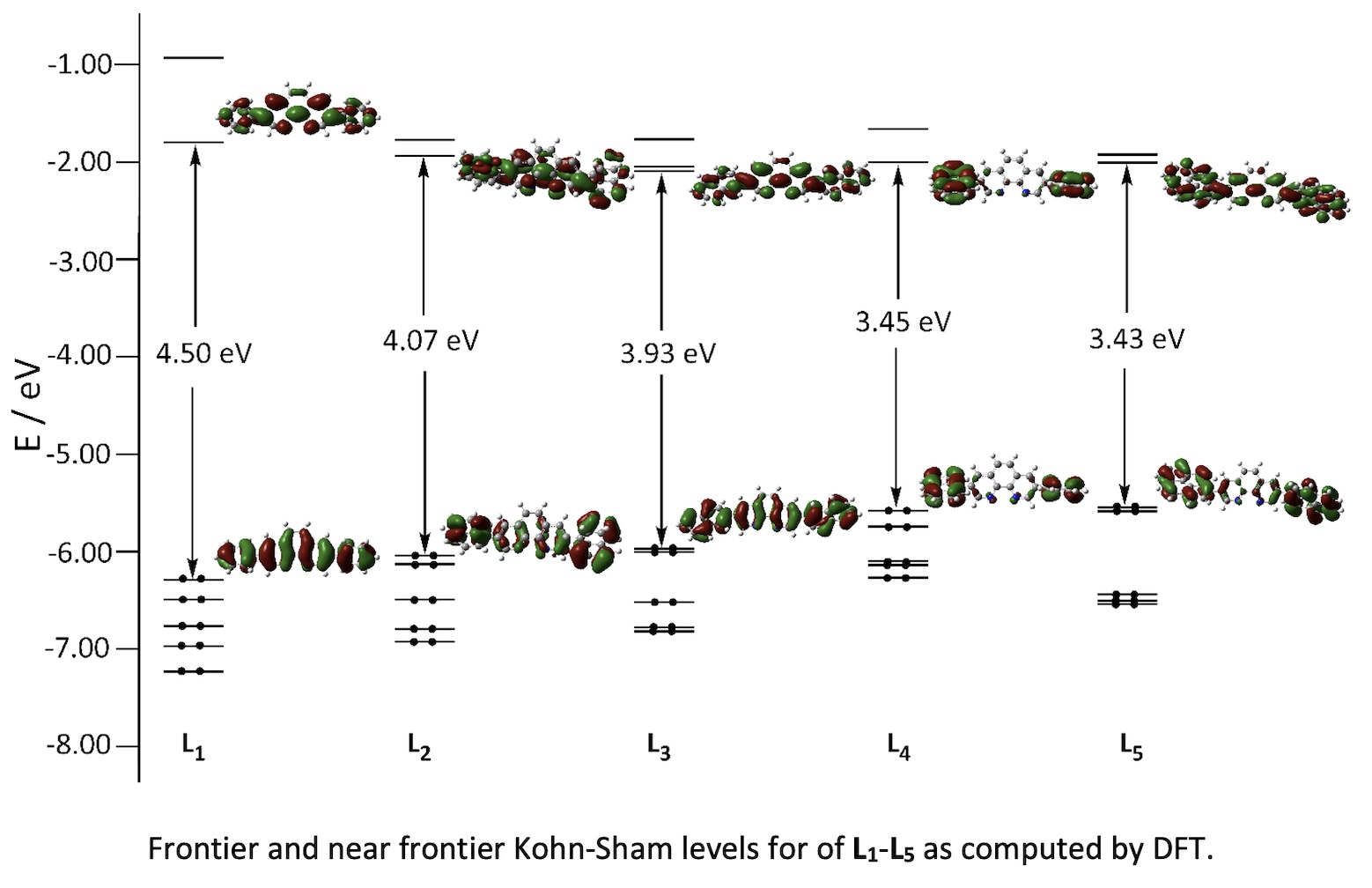
The molecules 3,8-diphenyl-1,10-phenanthroline (L1), 3,8-di(naphthalen-1-yl)-1,10-phenanthroline (L2), 3,8-di(naphthalen-2-yl)-1,10-phenanthroline (L3), 3,8-di(anthracen-9-yl)-1,10-phenanthroline (L4), and 3,8-di(pyren-1-yl)-1,10-phenanthroline (L5) were prepared in good yield from the reaction of 3,8-dibromo-1,10-phenanthroline with the corresponding boronic acid, catalysed by [Pd(PPh₃)₄]. Computational DFT modelling suggests that the aromatic substituent arms are not coplanar with the central phenanthroline (phen) core, and that the HOMO-LUMO gap diminishes as the number of fused carbon rings in the arms increases. Cyclic voltammograms for the ligands show between one and two oxidation and one to three reduction waves, which are believed to be centred on the arms and the central phen fragments, respectively, suggesting some small electronic mixing. The absorption and emission properties depend on the electronic interaction between the polyaromatic substituents and the phenanthroline core. Different emissive ππ* excited states for the molecules bearing anthryl and pyrenyl substituents suggest they have more charge transfer character, and a consequent sensitivity to the increase of solvent polarity. The photosensitizing capacity of singlet oxygen generation upon excitation of L1-L5 in solution is consistent with a significant evolution of the former singlet excited state towards a triplet excited state.
References
- Pashaei, B., et al., Polypyridyl ligands as a versatile platform for solid-state light-emitting devices. Chemical Society Reviews, 2019. 48(19): p. 5033-5139.
- Yip, A.M.-H. and K.K.-W. Lo, Luminescent rhenium(I), ruthenium(II), and iridium(III) polypyridine complexes containing a poly(ethylene glycol) pendant or bioorthogonal reaction group as biological probes and photocytotoxic agents. Coordination Chemistry Reviews, 2018. 361: p. 138-163.
- Martínez-Alonso, M. and G. Gasser, Ruthenium polypyridyl complex-containing bioconjugates. Coordination Chemistry Reviews, 2021. 434: p. 213736.
- Adeloye, A.O. and P.A. Ajibade, Towards the Development of Functionalized PolypyridineLigands for Ru(II) Complexes as Photosensitizers inDye-Sensitized Solar Cells (DSSCs). Molecules, 2014. 19(8): p. 12421-12460.
- Choroba, K., et al., Aryl substituted 2,6-di(thiazol-2-yl)pyridines –excited-state characterization and potential for OLEDs. Dyes and Pigments, 2019. 169: p. 89-104.
- Sravani Gudikandula, A.K., Navaneetha Nambigari, Application of Ru(II) polypyridyl complexes in metallopharmaceuticals and material science. Mediterranean Journal of Chemistry, 2023. 13(3): p. 263-284.
- Schramm, F., et al., Expanding the Coordination Cage: A Ruthenium(II)−Polypyridine Complex Exhibiting High Quantum Yields under Ambient Conditions. Inorganic Chemistry, 2009. 48(13): p. 5677-5684.
- Campagna, S., et al., Photochemistry and Photophysics of Coordination Compounds: Ruthenium, in Photochemistry and Photophysics of Coordination Compounds I, V. Balzani and S. Campagna, Editors. 2007, Springer Berlin Heidelberg: Berlin, Heidelberg. p. 117-214.
- Louërat, F. and P.C. Gros, Functional polypyridine ligands from copper-mediated room temperature coupling of 4-chloro-2-trimethylsilylpyridine. Tetrahedron Letters, 2010. 51(27): p. 3558-3560.
- Ferreira, H., M.M. Conradie, and J. Conradie, Cyclic voltammetry data of polypyridine ligands and Co(II)-polypyridine complexes. Data in Brief, 2019. 22: p. 436-445.
- Sammes, P.G. and G. Yahioglu, 1,10-Phenanthroline: a versatile ligand. Chemical Society Reviews, 1994. 23(5): p. 327-334.
- Bencini, A. and V. Lippolis, 1,10-Phenanthroline: A versatile building block for the construction of ligands for various purposes. Coordination Chemistry Reviews, 2010. 254(17): p. 2096-2180.
- Kulapina, E.G. and N.M. Makarova, Potentiometric Sensors Based on Various Active Components for the Multisensor Determination of Anionic and Nonionic Surfactants. Journal of Analytical Chemistry, 2022. 77(2): p. 173-184.
- Estalayo-Adrián, S., et al., Effect of Alkyl Chain Length on the Photophysical, Photochemical, and Photobiological Properties of Ruthenium(II) Polypyridyl Complexes for Their Application as DNA-Targeting, Cellular-Imaging, and Light-Activated Therapeutic Agents. ACS Applied Bio Materials, 2021. 4(9): p. 6664-6681.
- Uflyand, I.E. and G.I. Dzhardimalieva, Molecular design of supramolecular polymers with chelated units and their application as functional materials. Journal of Coordination Chemistry, 2018. 71(9): p. 1272-1356.
- Ding, X., H. Xie, and Y.J. Kang, The significance of copper chelators in clinical and experimental application. The Journal of Nutritional Biochemistry, 2011. 22(4): p. 301-310.
- Duce, J.A. and A.I. Bush, Biological metals and Alzheimer's disease: Implications for therapeutics and diagnostics. Progress in Neurobiology, 2010. 92(1): p. 1-18.
- S. Ressalan and C.S.P. Iyer, Review of Spectrofluorimetric Methods for the Determination of Copper, Nickel and Zinc. Reviews in Analytical Chemistry, 2004. 23(3): p. 159-232.
- Case, F.H. and R. Sasin, Substituted 1,10-phenanthrolines. VIII. 2- and 3-phenyl derivatives. The Journal of Organic Chemistry, 1955. 20(10): p. 1330-1336.
- Li, B., et al., Direct arylation of phenanthroline derivatives via oxidative C–H/C–H cross-coupling: synthesis and discovery of excellent ligands. Organic & Biomolecular Chemistry, 2013. 11(8): p. 1290-1293.
- Li, X., et al., Synthesis and characterization of novel rhenium(I) complexes with large Stokes shift for applications in organic electroluminescent devices. Journal of Photochemistry and Photobiology A: Chemistry, 2012. 241: p. 1-7.
- Ritter, K., et al., Optimized synthesis of a tert-butyl-phenyl-substituted tetrapyridophenazine ligand and its Ru(II) complexes and determination of dimerization behaviour of the complexes through supramolecular “Fingerhakel”. Dalton Transactions, 2015. 44(19): p. 8889-8905.
- Nishikawa, Y. and H. Yamamoto, Iron-Catalyzed Asymmetric Epoxidation of β,β-Disubstituted Enones. Journal of the American Chemical Society, 2011. 133(22): p. 8432-8435.
- Rothe, C., et al., Ionic Iridium(III) Complexes with Bulky Side Groups for Use in Light Emitting Cells: Reduction of Concentration Quenching. Advanced Functional Materials, 2009. 19(13): p. 2038-2044.
- Crosby, G.A. and J.N. Demas, Measurement of photoluminescence quantum yields. Review. The Journal of Physical Chemistry, 1971. 75(8): p. 991-1024.
- Suzuki, K., et al., Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector. Phys Chem Chem Phys, 2009. 11(42): p. 9850-60.
- Lakowicz, J.R., Principles of fluorescence spectroscopy. 2006, New York: Springer.
- Schmidt, R., et al., Phenalenone, a universal reference compound for the determination of quantum yields of singlet oxygen O2(1Δg) sensitization. Journal of Photochemistry and Photobiology A: Chemistry, 1994. 79(1-2): p. 11-17.
- Frisch, M.J.T., G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato et al., Gaussian 09. 2009, Gaussian, Inc.: Wallingford CT.
- Miertuš, S., E. Scrocco, and J. Tomasi, Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chemical Physics, 1981. 55(1): p. 117-129.
- Miertus̃, S. and J. Tomasi, Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chemical Physics, 1982. 65(2): p. 239-245.
- Sheppard, C.L., et al., Polynuclear copper(II) complexes with μ2-1,1-azide bridges. Structural and magnetic properties. Inorganica Chimica Acta, 1996. 250(1-2): p. 227-239.
- Humphrey, W., A. Dalke, and K. Schulten, VMD: visual molecular dynamics. J Mol Graph, 1996. 14(1): p. 33-8, 27-8.
- U. Florke, Y.V., M. Bauer., 3,8-bis(4-methylphenyl)-1,10-phenanthroline chloroform solvate. CSD Communication(Private Communication), 2018.
- Wang, X., et al., Europium(III) complex fluorescent sensor for dual channel recognition of Sn2+ and Cu2+ ions in water. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2021. 250: p. 119373.
- Doettinger, F., et al., Bichromophoric Photosensitizers: How and Where to Attach Pyrene Moieties to Phenanthroline to Generate Copper(I) Complexes. Inorganic Chemistry, 2023. 62(21): p. 8166-8178.
- Benazzi, E., et al., Bis(1,10-phenanthroline) copper complexes with tailored molecular architecture: from electrochemical features to application as redox mediators in dye-sensitized solar cells. Electrochimica Acta, 2018. 271: p. 180-189.
- Wantulok, J., et al., The effects of 4,7-di(pyrrolidin-1-yl) substituents on the reduction and oxidation mechanisms of 1,10-phenanthrolines: New perspectives in tailoring of phenantroline derivatives. Electrochimica Acta, 2021. 370: p. 137674.
- Chen, C., et al., Intramolecular Charge Transfer Controls Switching Between Room Temperature Phosphorescence and Thermally Activated Delayed Fluorescence. Angewandte Chemie International Edition, 2018. 57(50): p. 16407-16411.
- Li, P., et al., Intramolecular π Stacking in Cationic Iridium(III) Complexes with Phenyl-Functionalized Cyclometalated Ligands: Synthesis, Structure, Photophysical Properties, and Theoretical Studies. European Journal of Inorganic Chemistry, 2014. 2014(14): p. 2376-2382.
- Szlapa-Kula, A., M. Małecka, and B. Machura, Insight into structure-property relationships of aryl-substituted 2,2′:6′,2″-terpyridines. Dyes and Pigments, 2020. 180: p. 108480.
- Wilkinson, F., W.P. Helman, and A.B. Ross, Quantum Yields for the Photosensitized Formation of the Lowest Electronically Excited Singlet State of Molecular Oxygen in Solution. Journal of Physical and Chemical Reference Data, 1993. 22(1): p. 113-262.

