EXPLORING ARYL-SUBSTITUTED 1,2,3-TRIAZOLES: SYNTHESIS, CHARACTERIZATION, AND THEORETICAL INVESTIGATIONS

- 1,2,3-triazoles disubstituted,
- copper(I)-catalyzed,
- azide-alkyne cycloaddition,
- DFT calculations
Copyright (c) 2024 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
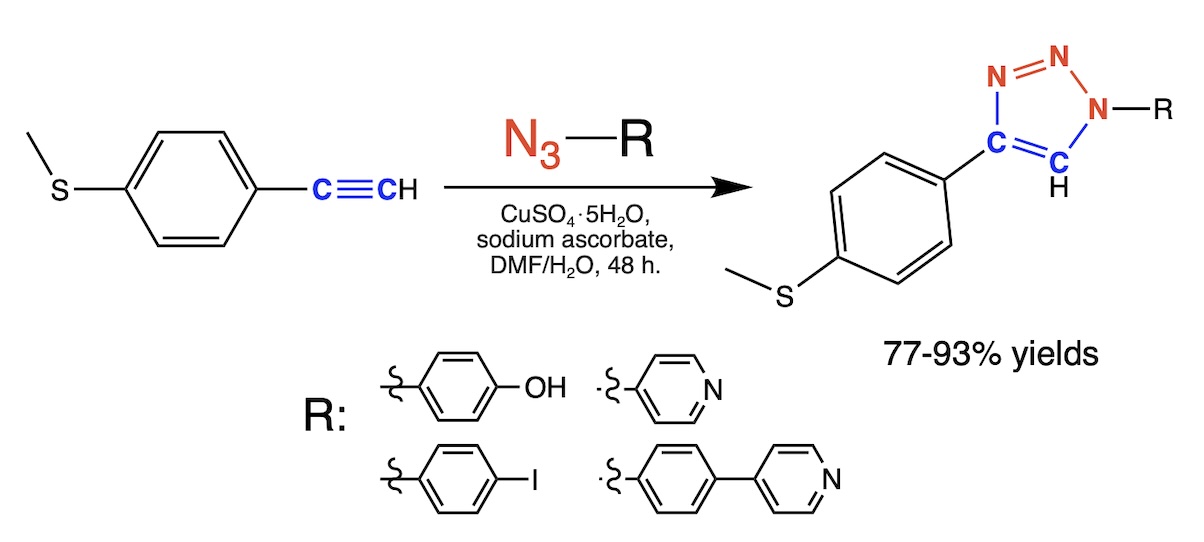
This study synthesized newly designed 1,2,3-triazoles substituted with aryl groups via Sharpless' copper(I)-catalyzed azide-alkyne cycloaddition. The resulting compounds were extensively characterized using NMR and UV-Vis spectroscopy. Furthermore, theoretical DFT and time-dependent DFT calculations were performed to analyze the structural and electronic properties of these molecules. Computational analysis revealed insights into the electron distribution within these molecules, with the electron-withdrawing or electron-donating nature of the substituents affecting the HOMO-LUMO gap. These findings provide valuable information for tailoring the electronic properties of triazole-containing compounds, making them suitable for various chemical applications and potential coordination with metalloporphyrins.
References
- (1) Dixit, D.; Verma, P. K.; Marwaha, R. K. A Review on ‘Triazoles’: Their Chemistry, Synthesis and Pharmacological Potentials. Journal of the Iranian Chemical Society 2021, 18 (10), 2535–2565. https://doi.org/10.1007/s13738-021-02231-x.
- (2) Hua, Y.; Flood, A. H. Click Chemistry Generates Privileged CH Hydrogen-Bonding Triazoles: The Latest Addition to Anion Supramolecular Chemistry. Chem Soc Rev 2010, 39 (4), 1262. https://doi.org/10.1039/b818033b.
- (3) Hein, J. E.; Fokin, V. V. Copper-Catalyzed Azide–Alkyne Cycloaddition (CuAAC) and beyond: New Reactivity of Copper(i) Acetylides. Chem Soc Rev 2010, 39 (4), 1302. https://doi.org/10.1039/b904091a.
- (4) Neto, J. S. S.; Zeni, G. A Decade of Advances in the Reaction of Nitrogen Sources and Alkynes for the Synthesis of Triazoles. Coord Chem Rev 2020, 409, 213217. https://doi.org/10.1016/j.ccr.2020.213217.
- (5) Mustafa, M.; Abdelhamid, D.; Abdelhafez, E. M. N.; Ibrahim, M. A. A.; Gamal-Eldeen, A. M.; Aly, O. M. Synthesis, Antiproliferative, Anti-Tubulin Activity, and Docking Study of New 1,2,4-Triazoles as Potential Combretastatin Analogues. Eur J Med Chem 2017, 141, 293–305. https://doi.org/10.1016/j.ejmech.2017.09.063.
- (6) Dehestani, L.; Ahangar, N.; Hashemi, S. M.; Irannejad, H.; Honarchian Masihi, P.; Shakiba, A.; Emami, S. Design, Synthesis, in Vivo and in Silico Evaluation of Phenacyl Triazole Hydrazones as New Anticonvulsant Agents. Bioorg Chem 2018, 78, 119–129. https://doi.org/10.1016/j.bioorg.2018.03.001.
- (7) Kaushik, C. P.; Pahwa, A. Convenient Synthesis, Antimalarial and Antimicrobial Potential of Thioethereal 1,4-Disubstituted 1,2,3-Triazoles with Ester Functionality. Medicinal Chemistry Research 2018, 27 (2), 458–469. https://doi.org/10.1007/s00044-017-2072-x.
- (8) Al-Masoudi, N. A.; Al-Soud, Y. A. Synthesis of 1′-β-d-Glucopyranosyl-1,2,3-Triazole-4,5-Dimethanol-4,5-Bis(Isopropylcarbamate) as Potential Antineoplastic Agent. Tetrahedron Lett 2002, 43 (22), 4021–4022. https://doi.org/10.1016/S0040-4039(02)00733-5.
- (9) Cao, X.; Wang, W.; Wang, S.; Bao, L. Asymmetric Synthesis of Novel Triazole Derivatives and Their in Vitro Antiviral Activity and Mechanism of Action. Eur J Med Chem 2017, 139, 718–725. https://doi.org/10.1016/j.ejmech.2017.08.057.
- (10) Sarigol, D.; Uzgoren-Baran, A.; Tel, B. C.; Somuncuoglu, E. I.; Kazkayasi, I.; Ozadali-Sari, K.; Unsal-Tan, O.; Okay, G.; Ertan, M.; Tozkoparan, B. Novel Thiazolo[3,2-b]-1,2,4-Triazoles Derived from Naproxen with Analgesic/Anti-Inflammatory Properties: Synthesis, Biological Evaluation and Molecular Modeling Studies. Bioorg Med Chem 2015, 23 (10), 2518–2528. https://doi.org/10.1016/j.bmc.2015.03.049.
- (11) Tantray, M. A.; Khan, I.; Hamid, H.; Alam, M. S.; Umar, S.; Ali, Y.; Sharma, K.; Hussain, F. Synthesis of Novel Oxazolo[4,5-b]Pyridine-2-One Based 1,2,3-Triazoles as Glycogen Synthase Kinase-3 β Inhibitors with Anti-Inflammatory Potential. Chem Biol Drug Des 2016, 87 (6), 918–926. https://doi.org/10.1111/cbdd.12724.
- (12) Safavi, M.; Ashtari, A.; Khalili, F.; Mirfazli, S. S.; Saeedi, M.; Ardestani, S. K.; Rashidi Ranjbar, P.; Barazandeh Tehrani, M.; Larijani, B.; Mahdavi, M. Novel Quinazolin-4(3 H )-One Linked to 1,2,3-Triazoles: Synthesis and Anticancer Activity. Chem Biol Drug Des 2018, 92 (1), 1373–1381. https://doi.org/10.1111/cbdd.13203.
- (13) Thakkar, S. S.; Thakor, P.; Doshi, H.; Ray, A. 1,2,4-Triazole and 1,3,4-Oxadiazole Analogues: Synthesis, MO Studies, in Silico Molecular Docking Studies, Antimalarial as DHFR Inhibitor and Antimicrobial Activities. Bioorg Med Chem 2017, 25 (15), 4064–4075. https://doi.org/10.1016/j.bmc.2017.05.054.
- (14) Nadeem, M.; Yunus, U.; Bhatti, M. H.; Ayub, K.; Mehmood, M.; Saif, M. J. Crystal Structure, Spectroscopic, Electronic, Luminescent and Nonlinear Optical Properties of (S)-4-Amino-5-(1-Hydroxy-Ethyl)-2,4-Dihydro-[1,2,4]Triazole-3-Thione: A Combined Experimental and DFT Study. Journal of Physics and Chemistry of Solids 2017, 110, 218–226. https://doi.org/10.1016/j.jpcs.2017.06.011.
- (15) Hempel, C.; Maichle-Mössmer, C.; Pericàs, M. A.; Nachtsheim, B. J. Modular Synthesis of Triazole-Based Chiral Iodoarenes for Enantioselective Spirocyclizations. Adv Synth Catal 2017, 359 (17), 2931–2941. https://doi.org/10.1002/adsc.201700246.
- (16) Rossi, R.; Bellina, F.; Lessi, M.; Manzini, C.; Perego, L. Synthesis of Multiply Arylated Heteroarenes, Including Bioactive Derivatives, via Palladium-Catalyzed Direct C–H Arylation of Heteroarenes with (Pseudo)Aryl Halides or Aryliodonium Salts. Synthesis (Stuttg) 2014, 46 (21), 2833–2883. https://doi.org/10.1055/s-0034-1378674.
- (17) Haldón, E.; Nicasio, M. C.; Pérez, P. J. Copper-Catalysed Azide–Alkyne Cycloadditions (CuAAC): An Update. Org Biomol Chem 2015, 13 (37), 9528–9550. https://doi.org/10.1039/C5OB01457C.
- (18) Kolb, H. C.; Sharpless, K. B. The Growing Impact of Click Chemistry on Drug Discovery. Drug Discov Today 2003, 8 (24), 1128–1137. https://doi.org/10.1016/S1359-6446(03)02933-7.
- (19) Pizarro, A.; Abarca, G.; Gutiérrez-Cerón, C.; Cortés-Arriagada, D.; Bernardi, F.; Berrios, C.; Silva, J. F.; Rezende, M. C.; Zagal, J. H.; Oñate, R.; Ponce, I. Building Pyridinium Molecular Wires as Axial Ligands for Tuning the Electrocatalytic Activity of Iron Phthalocyanines for the Oxygen Reduction Reaction. ACS Catal 2018, 8 (9), 8406–8419. https://doi.org/10.1021/acscatal.8b01479.
- (20) Fan, Y.; Pitie, S.; Liu, C.; Zhao, C.; Zhao, C.; Seydou, M.; Dappe, Y. J.; Nichols, R. J.; Yang, L. Asymmetric Effect on the Length Dependence of Oligo(Phenylene Ethynylene)-Based Molecular Junctions. The Journal of Physical Chemistry C 2022, 126 (7), 3635–3645. https://doi.org/10.1021/acs.jpcc.1c07654.
- (21) Gunderson, V. L.; Smeigh, A. L.; Kim, C. H.; Co, D. T.; Wasielewski, M. R. Electron Transfer within Self-Assembling Cyclic Tetramers Using Chlorophyll-Based Donor–Acceptor Building Blocks. J Am Chem Soc 2012, 134 (9), 4363–4372. https://doi.org/10.1021/ja211329k.
- (22) M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, M. C. X. Li, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A., D. J. F. Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016.
- (23) Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J Phys Chem 1994, 98 (45), 11623–11627. https://doi.org/10.1021/j100096a001.
- (24) Kim, K.; Jordan, K. D. Comparison of Density Functional and MP2 Calculations on the Water Monomer and Dimer. J Phys Chem 1994, 98 (40), 10089–10094. https://doi.org/10.1021/j100091a024.
- (25) Lee, C.; Yang, W.; Parr, R. G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys Rev B 1988, 37 (2), 785–789. https://doi.org/10.1103/PhysRevB.37.785.
- (26) Hehre, W. J.; Pau, C. Fong.; Headley, A. D.; Taft, R. W.; Topsom, R. D. A Scale of Directional Substituent Polarizability Parameters from Ab Initio Calculations of Polarizability Potentials. J Am Chem Soc 1986, 108 (7), 1711–1712. https://doi.org/10.1021/ja00267a063.
- (27) Marenich, A. V.; Cramer, C. J.; Truhlar, D. G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J Phys Chem B 2009, 113 (18), 6378–6396. https://doi.org/10.1021/jp810292n.
- (28) Chiodo, S.; Russo, N.; Sicilia, E. LANL2DZ Basis Sets Recontracted in the Framework of Density Functional Theory. J Chem Phys 2006, 125 (10). https://doi.org/10.1063/1.2345197.
- (29) An, S.; Meng, S.; Xue, J.; Wang, H.; Zheng, X.; Zhao, Y. UV–Vis, Raman Spectroscopic and Density Functional Theoretical Studies on Microsolvation 1, 2, 4-Triazole-3-Thione Clusters. Spectrochim Acta A Mol Biomol Spectrosc 2021, 258, 119762. https://doi.org/10.1016/j.saa.2021.119762.
- (30) Tankov, I.; Yankova, R. Hirshfeld Surface, DFT Vibrational (FT-IR) and Electronic (UV–Vis) Studies on 4-Amino-1H-1,2,4-Triazolium Nitrate. J Mol Struct 2019, 1179, 581–592. https://doi.org/10.1016/j.molstruc.2018.11.050.
- (31) Gökalp, M.; Dede, B.; Tilki, T.; Karabacak Atay, Ç. Triazole Based Azo Molecules as Potential Antibacterial Agents: Synthesis, Characterization, DFT, ADME and Molecular Docking Studies. J Mol Struct 2020, 1212, 128140. https://doi.org/10.1016/j.molstruc.2020.128140.
- (32) Zych, D.; Slodek, A. The Impact of a 1,2,3-Triazole Motif on the Photophysical Behavior of Non-K Tetrasubstituted Pyrene with a Substitution Pattern Providing the Long Axial Symmetry. Molecules 2022, 27 (13), 4314. https://doi.org/10.3390/molecules27134314.
- (33) Padalkar, V. S.; Lanke, S. K.; Chemate, S. B.; Sekar, N. N-2-Aryl-1,2,3-Triazoles: A Novel Class of Blue Emitting Fluorophores-Synthesis, Photophysical Properties Study and DFT Computations. J Fluoresc 2015, 25 (4), 985–996. https://doi.org/10.1007/s10895-015-1580-7.
- (34) Wang, T.-H.; Chu, H.-Y.; Wang, I.-T. Structures, Molecular Orbitals and UV–Vis Spectra Investigations on Methyl 1-Benzyl-1H-1,2,3-Triazole-4-Carboxylate: A Computational Study. Spectrochim Acta A Mol Biomol Spectrosc 2014, 131, 268–273. https://doi.org/10.1016/j.saa.2014.04.133.
- (35) Kim, T. Y.; Elliott, A. B. S.; Shaffer, K. J.; John McAdam, C.; Gordon, K. C.; Crowley, J. D. Rhenium(I) Complexes of Readily Functionalized Bidentate Pyridyl-1,2,3-Triazole “Click” Ligands: A Systematic Synthetic, Spectroscopic and Computational Study. Polyhedron 2013, 52, 1391–1398. https://doi.org/10.1016/j.poly.2012.05.003.
- (36) Tamer, Ö.; Bhatti, M. H.; Yunus, U.; Nadeem, M.; Avcı, D.; Atalay, Y.; Yaqub, A.; Quershi, R. Structure-Property Relationship of 3-(N-Phthalimidomethyl)-4-Amino-1,2,4-Triazole-5-Thione: A Structural, Spectroscopic and DFT Study. J Mol Struct 2017, 1133, 329–337. https://doi.org/10.1016/j.molstruc.2016.12.017.
- (37) Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J Comput Chem 2011, 32 (7), 1456–1465. https://doi.org/10.1002/jcc.21759.
- (38) Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J Comput Chem 2012, 33 (5), 580–592. https://doi.org/10.1002/jcc.22885.
- (39) Rukmanikrishnan, B.; Muthusamy, S. Preparation and Properties of Polyimides Containing 1,2,3-Triazole Moieties. Advances in Polymer Technology 2018, 37 (1), 50–59. https://doi.org/10.1002/adv.21641.
- (40) Gavlik, K. D.; Sukhorukova, E. S.; Shafran, Y. M.; Slepukhin, P. A.; Benassi, E.; Belskaya, N. P. 2-Aryl-5-Amino-1,2,3-Triazoles: New Effective Blue-Emitting Fluorophores. Dyes and Pigments 2017, 136, 229–242. https://doi.org/10.1016/j.dyepig.2016.08.015.
- (41) Săcărescu, L.; Dascălu, M.; Chibac-Scutaru, A.-L.; Roman, G. Synthesis, Structural Characterization, Photophysical Study and Investigation as Fluorescent Sensor towards Metal Ions of 1,2,3-Triazole–Azaindene Hybrids. J Photochem Photobiol A Chem 2022, 433, 114160. https://doi.org/10.1016/j.jphotochem.2022.114160.
- (42) Yan, W.; Wang, Q.; Lin, Q.; Li, M.; Petersen, J. L.; Shi, X. N-2-Aryl-1,2,3-Triazoles: A Novel Class of UV/Blue-Light-Emitting Fluorophores with Tunable Optical Properties. Chemistry - A European Journal 2011, 17 (18), 5011–5018. https://doi.org/10.1002/chem.201002937.
- (43) Laird, R. C.; Nguyen, N. P.; Rusch, S. F.; Baltrusaitis, J.; MacGillivray, L. R. Noncentrosymmetric Packings Influenced by Electronic Properties of Products of Click Reactions. Cryst Growth Des 2014, 14 (3), 893–896. https://doi.org/10.1021/cg4016542.
- (44) Singh, H.; Sindhu, J.; Khurana, J. M. Synthesis and Photophysical Properties of Novel Chloroquinoline Based Chalcone Derivates Containing 1,2,3-Triazole Moiety. J Lumin 2015, 158, 340–350. https://doi.org/10.1016/j.jlumin.2014.10.047.
- (45) Che, Y.; Perepichka, D. F. Quantifying Planarity in the Design of Organic Electronic Materials. Angewandte Chemie International Edition 2021, 60 (3), 1364–1373. https://doi.org/10.1002/anie.202011521.
- (46) Cheng, X.; Zhang, X.; Zhao, Y.; Zhuo, L. Theoretical Investigation of the Borazine B9N9 Monocyclic Ring. Chem Phys Lett 2023, 821, 140476. https://doi.org/10.1016/j.cplett.2023.140476.
- (47) Steinmann, S. N.; Mo, Y.; Corminboeuf, C. How Do Electron Localization Functions Describe π-Electron Delocalization? Physical Chemistry Chemical Physics 2011, 13 (46), 20584. https://doi.org/10.1039/c1cp21055f.

