AN OPTIMIZED KINETIC SPECTROPHOTOMETRIC METHOD FOR THE RAPID AND ACCURATE DETERMINATION OF CEFOPERAZONE IN URINE AND TAP WATER SAMPLES

- Cefoperazone sodium,
- Kinetic determination,
- Spectrophotometry
Copyright (c) 2024 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
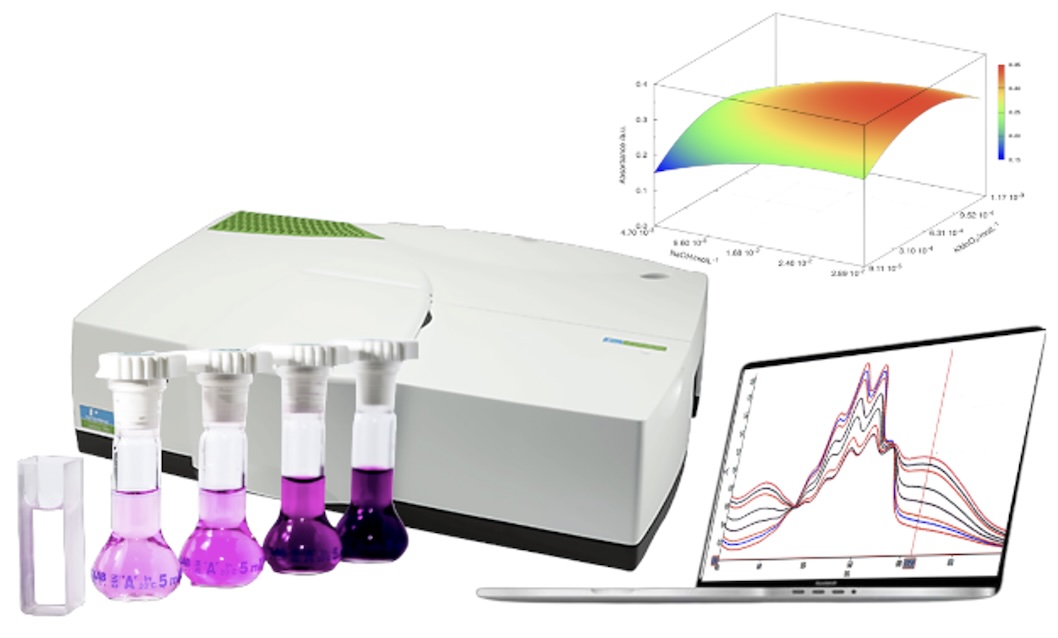
This study presents a new approach for quantifying the antibiotic cefoperazone (CPZ) in spiked urine and tap water samples using an indirect kinetic
spectrophotometry method. The method involves the oxidation of CPZ by permanganate (MnO4-) in an alkaline media. The progress of the reaction
is monitored by tracking the increase in manganate (MnO42- ) at 610 nm. The optimization of chemical-dependent variables was achieved through the utilization of multivariate statistical methods. The optimized values were 5 minutes of reaction time, KMnO4 of 1.11 · 10 -3 mol L−1, and NaOH of
0.27 mol L−1. Under these conditions, calibration curves were constructed. The detection limits obtained in urine and tap water spiked samples were
3.75 · 10−7 mol L−1 and 3.74 · 10−7 mol L−1, respectively. The results showed that intraday and interday recoveries ranged from 98.25 to 102.7%, indicating acceptable differences between observed and expected values according to the confidence percentage established as a criterion of acceptability; thus, demonstrating repeatability in results as well as satisfactory accuracy for this kinetic analytical method.
References
- References
- M. S. Cohen, H. E. Washton, S. F. Barranco, Multicenter clinical trial of cefopera- zone sodium in the united states, The American Journal of Medicine 77 (1, Part 2) (1984) 35 ? 41. doi:10.1016/S0002-9343(84)80094-7.
- H. S. Sader, C. G. Carvalhaes, J. M. Streit, M. Castanheira, R. K. Flamm, Antimi- crobial activity of cefoperazone-sulbactam tested against gram-negative organisms from europe, asia-pacific, and latin america, International Journal of Infectious Dis- eases 91 (2020) 32?37. doi:10.1016/j.ijid.2019.11.006.
- D. S. Wishart, C. Knox, A. C. Guo, S. Shrivastava, M. Hassanali, P. Stothard, Z. Chang, J. Woolsey, Drugbank: a comprehensive resource for in silico drug dis- covery and exploration, Nucleic Acids Research 34 (suppl. 1) (2006) D668?D672. doi:10.1093/nar/gkj067.
- USP 29, The United States Pharmacopeia : USP 29: The National Formulary : NF 24 : by Authority of the United States Pharmacopeial Convention, Meeting at Washington, D.C., March 9-13, 2005, Rockville, MD, 2006.
- L. Chen, O. Sha, W.-J. Zhai, J.-H. Yang, Fading spectrophotometric determina- tion of cefoperazone sodium in injections by Fe3+-sulfosalicylic acid system, Asian Journal of Chemistry 25 (14) (2013) 7918 ? 7920. doi:10.14233/ajchem.2013.14705.
- V. D. Hoang, N. T. Loan, V. T. Tho, H. M. T. Nguyen, UV spectrophoto- metric simultaneous determination of cefoperazone and sulbactam in pharmaceu- tical formulations by derivative, fourier and wavelet transforms, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 121 (2014) 704 ? 714. doi:10.1016/j.saa.2013.11.095.
- R. Ali, H. R. H. Ali, H. A. Batakoushy, S. M. Derayea, M. M. Elsutohy, A reductant colorimetric method for the rapid detection of certain cephalosporins via the pro- duction of gold and silver nanoparticles, Microchemical Journal 146 (2019) 864?871. doi:10.1016/j.microc.2019.02.023.
- H. A. B. Hassan Refat H. Ali, Ramadan Ali, S. M. Derayea, Spectroscopic analysis and antibacterial evaluation of certain third generation cephalosporins through metal complexation, Analytical Chemistry Letters 7 (4) (2017) 445?457. doi:10.1080/22297928.2017.1366868.
- Y. Yuan, S. Fu, Q. Xu, J. Yang, X. Hu, S. Liu, The fluorescence and resonance rayleigh scattering spectral study and analytical application of cerium (IV) and ce- foperazone system, Spectrochimica Acta Part A: Molecular and Biomolecular Spec- troscopy 162 (2016) 93 ? 97. doi:10.1016/j.saa.2016.03.001.
- M. E.-A. Sobhy, E. Mohamed, Hossinny, E. Sadek, H. H. Marwa, HPLC determi- nation of cefepime, cefotaxime and cefoperazone in bulk and dosage forms, Asian Journal of Pharmaceutical Analysis 4 (4) (2014) 168 ? 173.
- M. F. Al-Hakkani, N. Ahmed, A. A. Abbas, M. H. A. Hassan, Cefoperazone rapidly and sensitive quantitative assessment via a validated RP-HPLC method for different dosage forms, in-use stability, and antimicrobial activities, BMC Chemistry 17 (1) (2023) 72. doi:10.1186/s13065-023-00989-0.
- R.-H. Wang, L.-M. Cheng, H.-Y. Qu, G.-T. Hao, Z.-Y. Liu, H. Xia, Determina- tion of cefoperazone and sulbactam by LC-MS/MS in plasma and ultrafiltrate of patients undergone continuous renal replacement therapy, Journal of International Pharmaceutical Research 42 (2015) 199?205. doi:10.13220/j.cnki.jipr.2015.02.014.
- Y.-H. Gao, B.-Q. Li, X.-M. Cheng, A.-C. Liu, J.-G. Tian, Determination of the content of cefoperazone sodium and tazobactam sodium in dog plasma by liquid chromatography-tandem mass spectrometry, Chinese Journal of Pharmaceutical Analysis 25 (11) (2005).
- P. Norouzi, B. Larijani, F. Faridbod, M. R. Ganjali, Determination of cefoperazone based on nano-composite electrode using coulometric FFT admittance voltammetry, International Journal of Electrochemical Science 8 (5) (2013 ) 6118?6130.
- B.Dogan,A.Golcu,M.Dolaz,S.A.Ozkan,Electrochemicalbehaviourofthebacte- ricidal cefoperazone and its selective voltammetric determination in pharmaceutical dosage forms and human serum, Current Pharmaceutical Analysis 5 (2) (2009) 179? 189. doi:10.2174/157341209788172924.
- S. Billov , R. Kizek, F. Jelen, P. Novotn , Square-wave voltammetric determina- tion of cefoperazone in a bacterial culture, pharmaceutical drug, milk, and urine, Analytical and Bioanalytical Chemistry 377 (2003) 362 ? 369. doi:10.1007/s00216- 003-2109-5.
- V. D. Hoang, D. T. Huyen, P. H. Phuc, Adsorptive cathodic stripping voltammetric determination of cefoperazone in bulk powder, pharmaceutical dosage forms, and human urine, Journal of Analytical Methods in Chemistry 2013 (Article ID 367914) (2013). doi:10.1155/2013/367914.
- C. Thongpoon, B. Liawruangrath, S. Liawruangrath, R. A. Wheatley, A. Town- shend, Flow injection chemiluminescence determination of cephalosporins in pharmaceutical preparations using tris (2,2?-bipyridyl) ruthenium(II)-potassium permanganate system, Analytica Chimica Acta 553 (1) (2005) 123?133. doi:10.1016/j.aca.2005.07.056.
- G. A. Saleh, S. R. El-Shaboury, F. A. Mohamed, A. H. Rageh, Kinetic spectropho- tometric determination of certain cephalosporins using oxidized quercetin reagen, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 73 (5) (2009) 946?954. doi:10.1016/j.saa.2009.05.002.
- J. Du, H. Li, Sensitive chemiluminescence determination of thirteen cephalosporin antibiotics with luminol-copper(II) reaction, Applied Spectroscopy 64 (10) (2010) 1154 ? 1159. doi:10.1366/000370210792973613.
- A. M. El-Didamony, M. Z. Saad, D. S. El-Shaprawy, Direct and indirect spectropho- tometric determination of some selected antibiotics using potassium permanganate, Main Group Chemistry 12 (2013) 139 ? 152. doi:10.3233/MGC-130096.
- D. Y. Zhang, L. W. Zheng, Permanganate based chemiluminescence analysis of cefoperazon, in: Measurement Technology and Engineering Researches in Industry, Vol. 333 of Applied Mechanics and Materials, Trans Tech Publications Ltd, 2013, pp. 1807 ? 1810. doi:10.4028/www.scientific.net/AMM.333-335.1807.
- C. Soto, R. Saavedra, D. Contreras, C. Poza, S. Orellana, G. A. Olivares-Renter a, Flow injection analysis (FIA) with thermal lens spectrometry (TLS) for the rapid determination of cefoperazone in urine and water, Analytical Letters 55 (12) (2022) 1932 ? 1944. doi:10.1080/00032719.2022.2036181.
- L. G. Hargis, Analytical Chemistry: Principles and Techniques, Englewood Cliffs (N.J.): Prentice-Hall, 1988.

