Synthesis of Curcumin-ferulic acid conjugate via Steglich esterification and anti-lung cancer activity of against Human non-small lung cancer cells (NSLCC)
- Lung Cancer,
- Kinase,
- Apoptosis,
- cell-cycle
Copyright (c) 2024 JCChemS

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
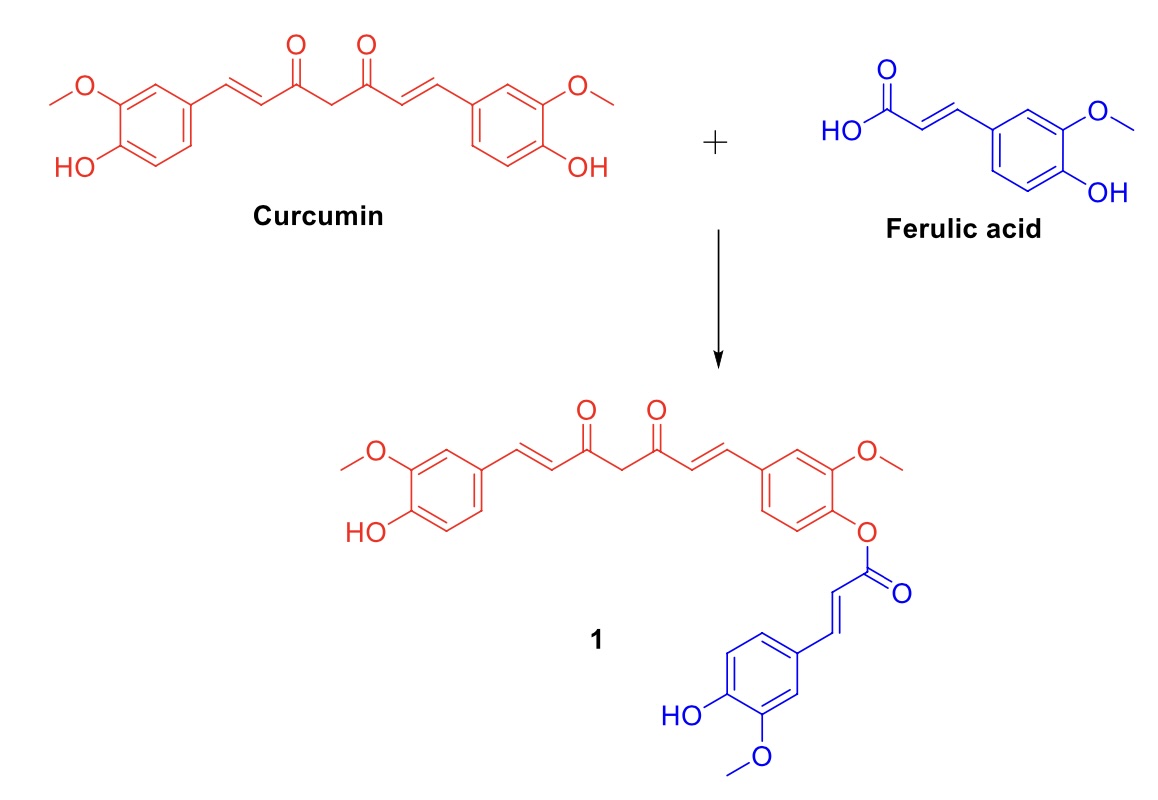
In the current work, the conjugate of curcumin and ferulic acid (compound 1) was developed and then examined utilizing a battery of biochemical assays to assess its pharmacological effectiveness against lung cancer. The compound 1 was synthesized using steglich esterification in excellent yield and then evaluated for its ability to inhibit the growth of different types of human cancer cells, including cells of the gastric cancer (SGC-7901), breast cancer (MCF7), liver cancer (HepG-2), lung cancer (A549), and human cervical carcinoma (HeLa). It exhibited stronger inhibitory effects on A549 cells compared to the other cell types, indicating its potent anti-lung cancer activity. It induces substantial suppression of many kinases, including EGFR, PI3K, mTOR, and VEGFR2. It exhibited cell cycle suppression of G2/M phase, resulting in a significant rise in apoptosis rate in A549 cells. Compound 1 showed a notable suppression of telomerase activity and a rise in the depolarization of mitochondrial membrane potential in A549 cells. The present study showcased the creation of a curcumin-ferulic acid conjugate (referred to as Compound 1) as a very potent anticancer medication that selectively targets lung cancer cells.
References
- Latimer KM, Mott TF. Lung cancer: Diagnosis, treatment principles, and screening. Am Fam Physician 2015. https://doi.org/d11787 [pii].
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209–49. https://doi.org/10.3322/caac.21660.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. https://doi.org/10.3322/caac.21492.
- Xiao Y, Liu P, Wei J, Zhang X, Guo J, Lin Y. Recent progress in targeted therapy for non-small cell lung cancer. Front Pharmacol 2023;14. https://doi.org/10.3389/fphar.2023.1125547.
- Wolf AMD, Oeffinger KC, Shih TY, Walter LC, Church TR, Fontham ETH, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin 2024;74. https://doi.org/10.3322/caac.21811.
- Kratzer TB, Bandi P, Freedman ND, Smith RA, Travis WD, Jemal A, et al. Lung cancer statistics, 2023. Cancer 2024;130. https://doi.org/10.1002/cncr.35128.
- Raskova Kafkova L, Mierzwicka JM, Chakraborty P, Jakubec P, Fischer O, Skarda J, et al. NSCLC: from tumorigenesis, immune checkpoint misuse to current and future targeted therapy. Front Immunol 2024;15. https://doi.org/10.3389/fimmu.2024.1342086.
- Lim JU. Update on Adjuvant Treatment in Resectable Non-Small Cell Lung Cancer and Potential Biomarkers Predicting Postoperative Relapse. Tuberc Respir Dis (Seoul) 2023;86. https://doi.org/10.4046/trd.2022.0081.
- Butler MS. Natural products to drugs: Natural product-derived compounds in clinical trials. Nat Prod Rep 2008;25:475–516. https://doi.org/10.1039/b514294f.
- Mang CP, Haustedt LO. Natural product scaffolds in cancer therapy. Natural Products and Cancer Drug Discovery, 2013, p. 123–73. https://doi.org/10.1007/978-1-4614-4654-5_6.
- Rayan A, Raiyn J, Falah M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS One 2017;12. https://doi.org/10.1371/journal.pone.0187925.
- Naeem A, Hu P, Yang M, Zhang J, Liu Y, Zhu W, et al. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022;27. https://doi.org/10.3390/molecules27238367.
- Hewlings SJ, Kalman DS. Curcumin: A review of its effects on human health. Foods 2017;6. https://doi.org/10.3390/foods6100092.
- Sun J, Chen F, Braun C, Zhou YQ, Rittner H, Tian YK, et al. Role of curcumin in the management of pathological pain. Phytomedicine 2018;48. https://doi.org/10.1016/j.phymed.2018.04.045.
- Pivari F, Mingione A, Brasacchio C, Soldati L. Curcumin and type 2 diabetes mellitus: Prevention and treatment. Nutrients 2019;11. https://doi.org/10.3390/nu11081837.
- Garodia P, Hegde M, Kunnumakkara AB, Aggarwal BB. Curcumin, inflammation, and neurological disorders: How are they linked? Integr Med Res 2023;12. https://doi.org/10.1016/j.imr.2023.100968.
- Ghosh S, Banerjee S, Sil PC. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food and Chemical Toxicology 2015;83. https://doi.org/10.1016/j.fct.2015.05.022.
- Giordano A, Tommonaro G. Curcumin and cancer. Nutrients 2019;11. https://doi.org/10.3390/nu11102376.
- Zoi V, Galani V, Lianos GD, Voulgaris S, Kyritsis AP, Alexiou GA. The role of curcumin in cancer treatment. Biomedicines 2021;9. https://doi.org/10.3390/biomedicines9091086.
- Tang X, Ding H, Liang M, Chen X, Yan Y, Wan N, et al. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac Cancer 2021;12. https://doi.org/10.1111/1759-7714.13904.
- He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015;20. https://doi.org/10.3390/molecules20059183.
- Li D, Rui Y xin, Guo S duo, Luan F, Liu R, Zeng N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci 2021;284. https://doi.org/10.1016/j.lfs.2021.119921.
- de Paiva LB, Goldbeck R, dos Santos WD, Squina FM. Ferulic acid and derivatives: Molecules with potential application in the pharmaceutical field. Brazilian Journal of Pharmaceutical Sciences 2013;49. https://doi.org/10.1590/S1984-82502013000300002.
- Marcato DC, Spagnol CM, Salgado HRN, Isaac VLB, Corrêa MA. New and potential properties, characteristics, and analytical methods of ferulic acid: A review. Brazilian Journal of Pharmaceutical Sciences 2022;58. https://doi.org/10.1590/s2175-97902020000118747.
- Li X, Wu J, Xu F, Chu C, Li X, Shi X, et al. Use of Ferulic Acid in the Management of Diabetes Mellitus and Its Complications. Molecules 2022;27. https://doi.org/10.3390/molecules27186010.
- Zhang X, Lin D, Jiang R, Li H, Wan J, Li H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol Rep 2016;36. https://doi.org/10.3892/or.2016.4804.
- Sin Singer Brugiolo A, Carvalho Gouveia AC, de Souza Alves CC, de Castro e Silva FM, Esteves de Oliveira É, Ferreira AP. Ferulic acid supresses Th2 immune response and prevents remodeling in ovalbumin-induced pulmonary allergy associated with inhibition of epithelial-derived cytokines. Pulm Pharmacol Ther 2017;45. https://doi.org/10.1016/j.pupt.2017.07.001.
- Neto-Neves EM, da Silva Maia Bezerra Filho C, Dejani NN, de Sousa DP. Ferulic Acid and Cardiovascular Health: Therapeutic and Preventive Potential. Mini-Reviews in Medicinal Chemistry 2021;21. https://doi.org/10.2174/1389557521666210105122841.
- Ohashi H, Tsuji M, Oguchi T, Momma Y, Nohara T, Ito N, et al. Combined Treatment with Curcumin and Ferulic Acid Suppressed the Aβ-Induced Neurotoxicity More than Curcumin and Ferulic Acid Alone. Int J Mol Sci 2022;23. https://doi.org/10.3390/ijms23179685.
- Paciello F, Rita Fetoni A, Mezzogori D, Rolesi R, Di Pino A, Paludetti G, et al. The dual role of curcumin and ferulic acid in counteracting chemoresistance and cisplatin-induced ototoxicity. Sci Rep 2020;10. https://doi.org/10.1038/s41598-020-57965-0.
- Law S, Lo C, Han J, Yang F, Leung AW, Xu C. Design, Synthesis and Characterization of Novel Curcumin Derivatives. Nat Prod Chem Res 2020;8. https://doi.org/10.35248/2329-6836.20.8.367.
- Zhang Q, Hui M, Chen G, Huang H, Wang S, Ye Y, et al. Curcumin-Piperlongumine Hybrid Molecule Increases Cell Cycle Arrest and Apoptosis in Lung Cancer through JNK/c-Jun Signaling Pathway. J Agric Food Chem 2024;72. https://doi.org/10.1021/acs.jafc.4c00882.
- Hernández C, Moreno G, Herrera-R A, Cardona-G W. New hybrids based on curcumin and resveratrol: Synthesis, cytotoxicity and antiproliferative activity against colorectal cancer cells. Molecules 2021;26. https://doi.org/10.3390/molecules26092661.
- Serafim TL, Carvalho FS, Marques MPM, Calheiros R, Silva T, Garrido J, et al. Lipophilic caffeic and ferulic acid derivatives presenting cytotoxicity against human breast cancer cells. Chem Res Toxicol 2011;24. https://doi.org/10.1021/tx200126r.
- Le X, Nilsson M, Goldman J, Reck M, Nakagawa K, Kato T, et al. Dual EGFR-VEGF Pathway Inhibition: A Promising Strategy for Patients With EGFR-Mutant NSCLC. Journal of Thoracic Oncology 2021;16. https://doi.org/10.1016/j.jtho.2020.10.006.
- Zhang Z, Stiegler AL, Boggon TJ, Kobayashi S, Halmos B. EGFR-mutated lung cancer: a paradigm of molecular oncology. Oncotarget 2010;1. https://doi.org/10.18632/oncotarget.186.
- Qiao M, Jiang T, Liu X, Mao S, Zhou F, Li X, et al. Immune Checkpoint Inhibitors in EGFR-Mutated NSCLC: Dusk or Dawn? Journal of Thoracic Oncology 2021;16. https://doi.org/10.1016/j.jtho.2021.04.003.
- Garcia-Echeverria C, Sellers WR. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene 2008;27:5511–26. https://doi.org/10.1038/onc.2008.246.
- Fumarola C, Bonelli MA, Petronini PG, Alfieri RR. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem Pharmacol 2014. https://doi.org/10.1016/j.bcp.2014.05.011.
- Jiang L, Zhang J, Xu Y, Xu H, Wang M. Treating non-small cell lung cancer by targeting the PI3K signaling pathway. Chin Med J (Engl) 2022;135. https://doi.org/10.1097/CM9.0000000000002195.
- Mabeta P, Steenkamp V. The VEGF/VEGFR Axis Revisited: Implications for Cancer Therapy. Int J Mol Sci 2022;23. https://doi.org/10.3390/ijms232415585.
- Zhao Y, Guo S, Deng J, Shen J, Du F, Wu X, et al. VEGF/VEGFR-Targeted Therapy and Immunotherapy in Non-small Cell Lung Cancer: Targeting the Tumor Microenvironment. Int J Biol Sci 2022;18. https://doi.org/10.7150/ijbs.70958.
- Wang Q, Zeng A, Zhu M, Song L. Dual inhibition of EGFR VEGF: An effective approach to the treatment of advanced non small cell lung cancer with EGFR mutation (Review). Int J Oncol 2023;62. https://doi.org/10.3892/ijo.2023.5474.
- Lansdorp PM. Telomeres, Telomerase and Cancer. Arch Med Res 2022;53. https://doi.org/10.1016/j.arcmed.2022.10.004.
- Tian X, Chen B, Liu X. Telomere and telomerase as targets for cancer therapy. Appl Biochem Biotechnol 2010;160. https://doi.org/10.1007/s12010-009-8633-9.
- Elmore S. Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol 2007;35:495–516. https://doi.org/10.1080/01926230701320337.
- Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis 2000;21:485–95. https://doi.org/10.1093/carcin/21.3.485.
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature 2001. https://doi.org/10.1038/35077213.
- Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (δψm) in apoptosis; an update. Apoptosis 2003;8:115–28. https://doi.org/10.1023/A:1022945107762.
- Lopez J, Tait SWG. Mitochondrial apoptosis: Killing cancer using the enemy within. Br J Cancer 2015;112. https://doi.org/10.1038/bjc.2015.85.
- Liu Y, Lu S, Wu L lei, Yang L, Yang L, Wang J. The diversified role of mitochondria in ferroptosis in cancer. Cell Death Dis 2023;14. https://doi.org/10.1038/s41419-023-06045-y.
- Srivastava JK, Pillai GG, Bhat HR, Verma A, Singh UP. Design and discovery of novel monastrol-1,3,5-triazines as potent anti-breast cancer agent via attenuating Epidermal Growth Factor Receptor tyrosine kinase. Sci Rep 2017;7:5851. https://doi.org/10.1038/s41598-017-05934-5.
- Darekar SD, Mushtaq M, Gurrapu S, Kovalevska L, Drummond C, Petruchek M, et al. Mitochondrial ribosomal protein S18-2 evokes chromosomal instability and transforms primary rat skin fibroblasts. Oncotarget 2015;6. https://doi.org/10.18632/oncotarget.4123.
- Wang Z, Zuo J, Zhang L, Zhang Z, Wei Y. Plantamajoside promotes metformin-induced apoptosis, autophagy and proliferation arrest of liver cancer cells via suppressing Akt/GSK3β signaling. Hum Exp Toxicol 2022;41. https://doi.org/10.1177/09603271221078868.
- Hao M, Zhang K, Wang H, Wang J, Li J, Cheng R, et al. Paeonol Inhibits Glioma Growth In Vivo and In Vitro by Inducing Apoptosis and Cell Cycle Arrest. Revista Brasileira de Farmacognosia 2023;33. https://doi.org/10.1007/s43450-023-00380-z.
- Du J, Zhao Y, Hu D, Li H, Gao L, Xing X, et al. Pinosylvin Inhibits Esophageal Squamous Cell Carcinoma Migration and Invasion by Regulating STX6/ITGA3/VASP Pathway. Revista Brasileira de Farmacognosia 2023;33. https://doi.org/10.1007/s43450-022-00354-7.
- Ning Y, Fu Y ling, Zhang QH, Zhang C, Chen Y. Inhibition of in vitro and in vivo ovarian cancer cell growth by pinoresinol occurs by way of inducing autophagy, inhibition of cell invasion, loss of mitochondrial membrane potential and inhibition Ras/MEK/ERK signalling pathway. Journal of BUON 2019;24.

