Vol 69 No 1 (2024): Journal of the Chilean Chemical
Original Research Papers

Published
October 6, 2024
Keywords
- Lipoic acid,
- Steglich esterification,
- Lipoic derivatives
How to Cite
González-Gutiérrez, J. P., Moris, S., & Barrientos, C. (2024). Synthesis of novel lipoic acid derivatives. Journal of the Chilean Chemical Society, 69(1), 6054-6055. Retrieved from https://jcchems.com/index.php/JCCHEMS/article/view/2694
Copyright (c) 2024 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Lipoic acid is a naturally occurring compound involved in biological processes with special reactivity due to its 1,2-dithiolane ring. The novel ALA derivatives were synthesized using the Steglich esterification in mild conditions and then concentrated and neutralized with yields of 45.1 to 81.2%.
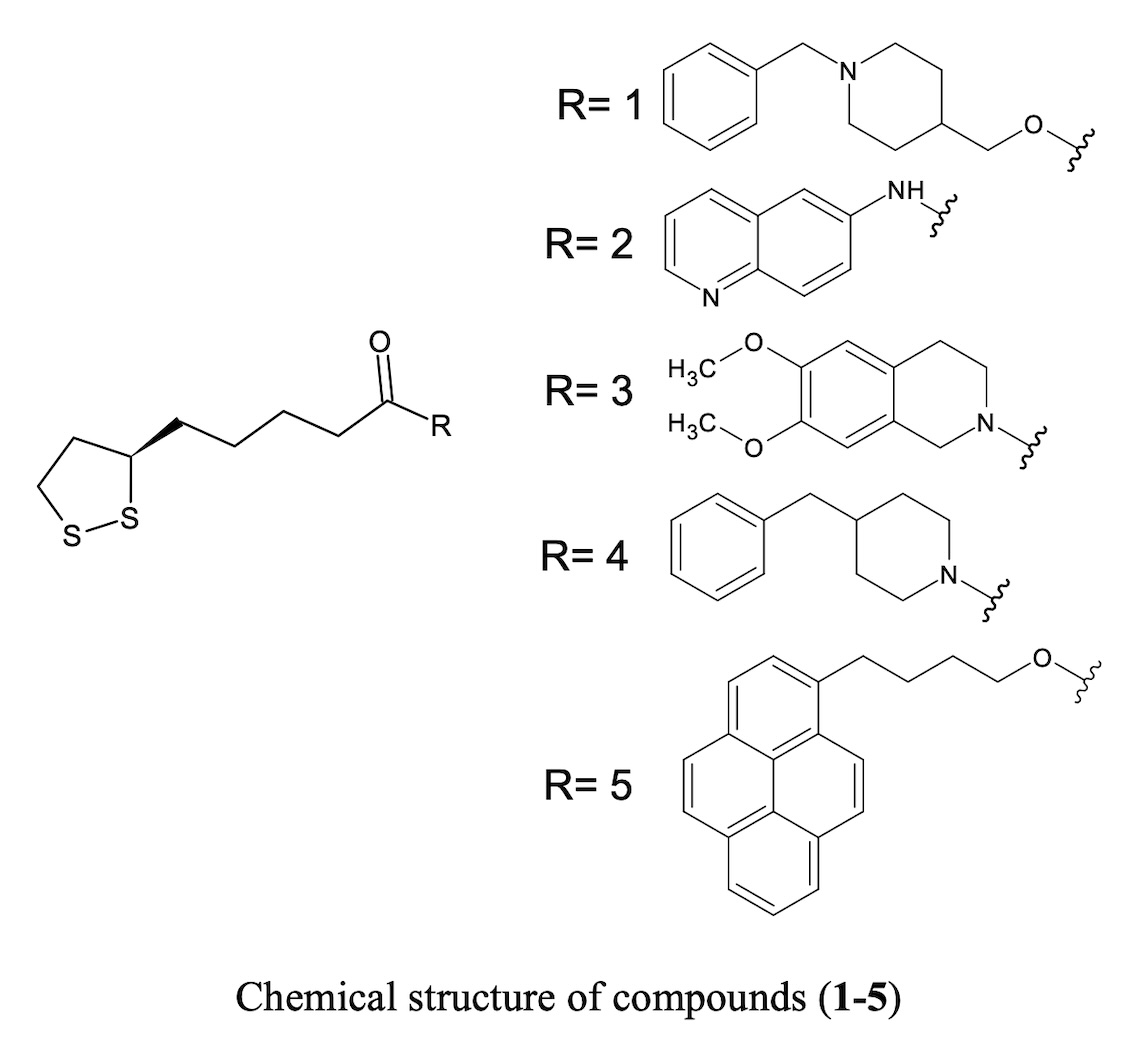
References
- C. Bellini, F. Mancin, E. Papini, R. Tavano, Antioxidants. 13 (2024).
- A.K. Tripathi, A.K. Ray, S.K. Mishra, S.M. Bishen, H. Mishra, A. Khurana, Rev. Bras. Farmacogn. 33 (2023).
- A. Gruzman, A. Hidmi, J. Katzhendler, A. Haj-Yehie, S. Sasson, Bioorg. Med. Chem. 12 (2004).
- M. Turkowicz, I. Jastrzebska, M. Hryniewicka, U. Kotowska, D. Gudalewska, J. Karpińska, Food Chem. 309 (2020).
- V.E. Kagan, E.A. Serbinova, G.M. Koynova, S.A. Kitanova, V.A. Tyurin, T.S. Stoytchev, P.J. Quinn, L. Packer, Free Radic. Biol. Med. 9 (1990).
- H. Xie, X. Yang, Y. Cao, X. Long, H. Shang, Z. Jia, CNS Neurosci. Ther. 28 (2022).
- S. Yan, J. Lu, B. Chen, L. Yuan, L. Chen, L. Ju, W. Cai, J. Wu, Antioxidants. 13 (2024).
- T. Aiba, Y. Kono, T. Etoh, Y. Kawano, Y. Oshima, M. Inomata, Cancer Sci. 114 (2023).
- M. Attia, E.A. Essa, R.M. Zaki, A.A. Elkordy, Antioxidants. 9 (2020).
- I. V Ozhogin, P. V Zolotukhin, V. V Tkachev, A.D. Pugachev, A.S. Kozlenko, A.A. Belanova, S.M. Aldoshin, B.S. Lukyanov, Russ. Chem. Bull. 70 (2021).
- D. Kaur, T. Behl, A. Sehgal, S. Singh, N. Sharma, S. Chigurupati, A. Alhowail, A. Abdeen, S.F. Ibrahim, C. Vargas-De-La-Cruz, M. Sachdeva, S. Bhatia, A. Al-Harrasi, S. Bungau, Life Sci. 284 (2021).
- D. Kong, A.A. Saqer, M. Carpinelli de Jesus, N. Khan, A. Jones, J.T. Blanchfield, M.T. Smith, C.M. Williams, Bioorg. Med. Chem. 69 (2022).
- A.C.F. Santos, R.C.S. Luz, F.S. Damos, A.E.G. Santana, D.G. Pessoa, M. Navarro, M.O.F. Goulart, J. Solid State Electrochem. 24 (2020).
- B.B. Petković, M. Ognjanović, B. Antić, V. Viktorovich Avdin, D.D. Manojlović, S. Vranješ Đurić, D.M. Stanković, Electroanalysis. 33 (2021).
- Y. Qian, L. Zhang, Y. Tian, Anal. Chem. 94 (2022).
- S. Lechner, R.R. Steimbach, L. Wang, M.L. Deline, Y.-C. Chang, T. Fromme, M. Klingenspor, P. Matthias, A.K. Miller, G. Médard, B. Kuster, Nat. Commun. 14 (2023).
- I.O. Levkovskyi, S. Mochizuki, A. Zheng, X. Zhang, F. Zhang, Nano TransMed. 2 (2023).
- Q. Yu, Z. Fang, S. Luan, L. Wang, H. Shi, J. Mater. Chem. B. 12 (2024).
- J. Gao, Q. Zhang, B. Wu, X. Gao, Z. Liu, H. Yang, J. Yuan, J. Huang, Small. 19 (2023).
- A. Ghosh, K. Kozlowski, T.W.J. Steele, Polymers (Basel). 15 (2023).
- R.M. Annuur, D. Triana, T. Ernawati, Y. Murai, M. Aswad, M. Hashimoto, Z.P. Tachrim, Molecules. 29 (2024).
- D. Olender, M. Józkowiak, H. Piotrowska-Kempisty, K. Sowa-Kasprzak, L. Zaprutko, I. Muszalska-Kolos, E. Baranowska-Wójcik, D. Szwajgier, Pharmaceuticals. 16 (2023).
- E. Popa, A.A. Andelescu, V. Badea, P. Svera (m. Ianăşi), E.I. Szerb, Molbank. 2024 (2024).


