FIRST REPORT ON THE BIOLOGICAL ACTIVITIES, MOLECULAR DOCKING AND STUDY OF THE TOXICITY OF TWO OLEORESINS AS WELL AS THEIR MAIN CONSTITUENTS

- Biological activities,
- molecular docking,
- Myrrh,
- Pharmacokinetics,,
- Pine resin
- Toxicity. ...More
Copyright (c) 2024 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
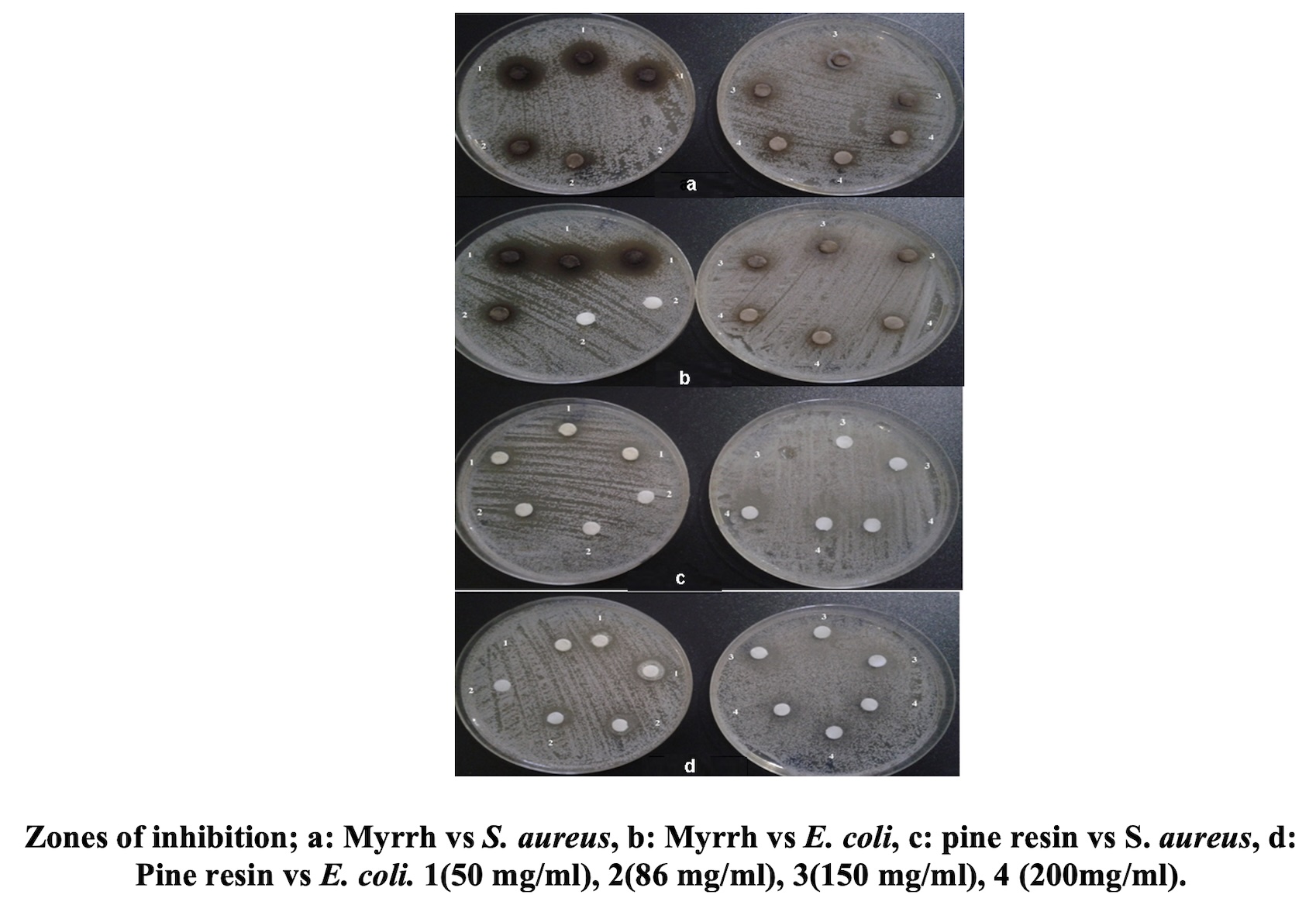
The objective of this work was to evaluate the in vitro and in silico antibacterial, anti-inflammatory and antioxidant activities of two oleoresins; Myrrh and Pine resin used in the Algerian traditional pharmacopoeia. The antibacterial effect of oleoresins was evaluated by the agar diffusion test against three bacterial strains; E. coli (ATCC 25922), S. aureus (ATCC 25923) and P. aeuroginosa (ATCC 27853). The antioxidant activity was assessed using DPPH method and the protein inhibition denaturation test was used to evaluate the anti-inflammatory efficacy. Resins main compounds were docked in silico against the bacterial tyrosyl-tRNA synthetase using the Autodock Tools 1.5.7 software. This study was carried out to determine their modes of binding with the active residues of this molecular target enzyme of antimicrobial agents. Molinspiration Cheminformatics and SwissADME online tools were used to predict physicochemical and pharmacokinetic parameters while OSIRIS Property Explorer online tools were used to predict toxicity risks. The results show that the Myrrh was effective against E. coli and S. aureus (17 mm) and that the Pine resin was similarly effective against E. coli (11 mm) and S. aureus (10 mm), but P. aeruginosa was completely resistant. The antioxidant test showed that both oleoresins had considerable ability to reduce the DPPH, with good IC50 of 0.49 ± 0.13 and 0.53 ± 0.06 mg/ml, respectively, compared to the BHT (0.89 ± 0.45 mg/ml). Both oleoresins had a remarkable anti-denaturation effects. The data of in silico studies revealed that all phytocompounds fit into the active pocket of the target enzyme and the binding energies ranged between -10.06 (Dehydroabietic acid) and -4.3 kcal/mol (D-glucuronic acid). The toxic and pharmacokinetic characteristics are, mostly, satisfying except for some compounds which have shown toxic effects, in particular Limonene, 4-allylanisole and Vanillin. We conclude that the extracts and their primary phytocompounds can enhance the antibacterial, antioxidant, and anti-inflammatory existing drugs without side effects.
References
- M. Zaynab, M. Fatima, S. Abbas, Y. Sharif, M. Umair, M. H. Zafar, K. Bahadar, Microb. Pathog. 124 (2018) 198–202. https://doi.org/10.1016/j.micpath.2018.08.034
- K. Rajashri, S. Mudhol, M. Serva Peddha, B. B. Borse, ACS Omega 5 (2020) 30898–30905. https://doi.org/10.1021/acsomega.0c03689
- F. Abrão, T. S. Silva, C. L. Moura, S. R. Ambrósio, R. C. S. Veneziani, R. E. F. de Paiva, J. K. Bastos, C. H. G. Martins, Sci. Rep. 11 (2021) 4953. https://doi.org/10.1038/s41598-021-84480-7
- M. Rubini, A. Clopeau, J. Sandak, S. Dumarcay, A. Sandak, P. Gerardin, B. Charrier, Biocatal. Agric. Biotechnol. 42 (2022) 102340. https://doi.org/10.1016/j.bcab.2022.102340
- C. Arrabal, M. Cortijo, B. F. de Simón, M. C. García Vallejo, E. Cadahía, Biochem. Syst. Ecol. 33 (2005) 1007–1016. https://doi.org/10.1016/j.bse.2005.03.003
- F. R. Procopio, M. C. Ferraz, B. N. Paulino, P. J. do Amaral Sobral, M. D. Hubinger, Trends Food Sci. Technol. 122 (2022) 123–139. https://doi.org/10.1016/j.tifs.2022.02.010
- E. Bolskis, E. Adomavičiūtė, E. Griškonis, V. Norvydas, Materials 13 (2020) 3824. https://doi.org/10.3390/ma13173824
- N. S. Younis, M. E. Mohamed, J. Ethnopharmacol. 270 (2021) 113793. https://doi.org/10.1016/j.jep.2021.113793
- M. A. Lebda, R. E. Mostafa, N. M. Taha, E. M. Abd El-Maksoud, H. G. Tohamy, S. K. Al Jaouni, A. H. El-Far, M. S. Elfeky, Antioxidants 10 (2021) 1836. https://doi.org/10.3390/antiox10111836
- H. N. Murthy, ed., Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses, Springer International Publishing, Cham, 2022. https://doi.org/10.1007/978-3-030-91378-6
- A. S. Alqahtani, R. N. Herqash, O. M. Noman, Md. Tabish Rehman, A. A. Shahat, M. F. Alajmi, F. A. Nasr, J. Anal. Methods Chem. 2021 (2021) 1–10. https://doi.org/10.1155/2021/5525173
- Z. Alehaideb, G. Alatar, A. Nehdi, A. Albaz, H. Al-Eidi, M. Almutairi, E. Hawsa, N. Alshuail, S. Matou-Nasri, Saudi Pharm. J. 29 (2021) 361–368. https://doi.org/10.1016/j.jsps.2021.03.002
- B. Cao, X.-C. Wei, X.-R. Xu, H.-Z. Zhang, C.-H. Luo, B. Feng, R.-C. Xu, S.-Y. Zhao, X.-J. Du, L. Han, D.-K. Zhang, Molecules 24 (2019) 3076. https://doi.org/10.3390/molecules24173076
- O. Ajiteru, O. J. Lee, J.-H. Kim, Y. J. Lee, J. S. Lee, H. Lee, Md. T. Sultan, C. H. Park, Colloid Interface Sci. Commun. 48 (2022) 100617. https://doi.org/10.1016/j.colcom.2022.100617
- H. Derdar, G. R. Mitchell, V. S. Mahendra, M. Benachour, S. Haoue, Z. Cherifi, K. Bachari, A. Harrane, R. Meghabar, Polymers 12 (2020) 1971. https://doi.org/10.3390/polym12091971
- N. Kadri, B. Khettal, Y. Aid, S. Kherfellah, W. Sobhi, V. Barragan-Montero, Food Chem. 188 (2015) 184–192. https://doi.org/10.1016/j.foodchem.2015.04.138
- N. Boulâacheb, Acta Hortic. (2010) 435–438. https://doi.org/10.17660/ActaHortic.2010.853.53
- A. P. Acosta, K. T. Barbosa, S. C. Amico, A. L. Missio, R. de Avila Delucis, D. A. Gatto, Ind. Crops Prod. 166 (2021) 113495. https://doi.org/10.1016/j.indcrop.2021.113495
- N. Garcia-Forner, F. Campelo, A. Carvalho, J. Vieira, A. Rodríguez-Pereiras, M. Ribeiro, A. Salgueiro, M. E. Silva, J. L. Louzada, For. Ecol. Manag. 496 (2021) 119406. https://doi.org/10.1016/j.foreco.2021.119406
- Z. Yaniv, Medicinal and aromatic plants of the Middle-East, Springer, New York, 2014.
- J. Y. Park, Y. K. Lee, D.-S. Lee, J.-E. Yoo, M.-S. Shin, N. Yamabe, S.-N. Kim, S. Lee, K. H. Kim, H.-J. Lee, S. S. Roh, K. S. Kang, J. Ethnopharmacol. 203 (2017) 279–287. https://doi.org/10.1016/j.jep.2017.03.055
- M. Skupińska, P. Stępniak, I. Łętowska, L. Rychlewski, M. Barciszewska, J. Barciszewski, M. Giel-Pietraszuk, Microb. Drug Resist. 23 (2017) 308–320. https://doi.org/10.1089/mdr.2015.0272
- C. A. Hughes, V. Gorabi, Y. Escamilla, F. B. Dean, J. M. Bullard, SLAS Discov. 25 (2020) 1072–1086. https://doi.org/10.1177/2472555220934793
- G. Bouz, J. Zitko, Bioorganic Chem. 110 (2021) 104806. https://doi.org/10.1016/j.bioorg.2021.104806
- EUCAST, (2022). Antimicrobial susceptibility testing The European Committee on Antimicrobial Susceptibility Testing.https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2022_manuals/Manual_v_10.0_EUCAST_Disk_Test_2022.pdf.
- G. Singh, P. Marimuthu, C. S. de Heluani, C. A. N. Catalan, J. Agric. Food Chem. 54 (2006) 174–181. https://doi.org/10.1021/jf0518610
- Reshma, P. BRINDHA, ARUN KP, 7 (2014) 9.
- L. O. Hanuš, T. Řezanka, V. M. Dembitsky, A. Moussaieff, Biomed. Pap. 149 (2005) 3–28. https://doi.org/10.5507/bp.2005.001
- N. S. Al-Radadi, Saudi Pharm. J. (2022) S1319016422001852. https://doi.org/10.1016/j.jsps.2022.06.028
- J. Ulrich, S. Stiltz, A. St-Gelais, M. El Gaafary, T. Simmet, T. Syrovets, M. Schmiech, Molecules 27 (2022) 3903. https://doi.org/10.3390/molecules27123903
- M. M. Zerroug, N. Haichour, S. Mezaache Aichour, E. Soltani, S. Kada, J. R. Martinez, M. Angeles Esteban, J. Nicklin, J. Microbiol. Biotechnol. Food Sci. 11 (2021) e3423. https://doi.org/10.15414/jmbfs.3423
- E. Mita, C. Tsitsimpikou, L. Tsiveleka, P. V. Petrakis, A. Ortiz, C. Vagias, V. Roussis, Holzforschung 56 (2002) 572–578. https://doi.org/10.1515/HF.2002.087
- F. A. Neis, F. de Costa, M. R. de Almeida, L. C. Colling, C. F. de Oliveira Junkes, J. P. Fett, A. G. Fett-Neto, Ind. Crops Prod. 132 (2019) 76–83. https://doi.org/10.1016/j.indcrop.2019.02.013
- A. Sukarno, Sutarman, Y. Q. Mondiana, D. W. Irawan, Y. A. Wiranegara, M. Abror, IOP Conf. Ser. Earth Environ. Sci. 1104 (2022) 012016. https://doi.org/10.1088/1755-1315/1104/1/012016
- D. Salaria, R. Rolta, C. N. Patel, K. Dev, A. Sourirajan, V. Kumar, J. Biomol. Struct. Dyn. (2021) 1–20. https://doi.org/10.1080/07391102.2021.1943530
- C. A. Lipinski, Drug Discov. Today Technol. 1 (2004) 337–341. https://doi.org/10.1016/j.ddtec.2004.11.007
- L. Adjissi, N. Chafai, K. Benbouguerra, I. Kirouani, A. Hellal, H. Layaida, M. Elkolli, C. Bensouici, S. Chafaa, J. Mol. Struct. 1270 (2022) 134005. https://doi.org/10.1016/j.molstruc.2022.134005
- P. Yoganantharajah, A. P. Ray, D. J. Eyckens, L. C. Henderson, Y. Gibert, BMC Biotechnol. 18 (2018) 32. https://doi.org/10.1186/s12896-018-0442-1
- J. Galvao, B. Davis, M. Tilley, E. Normando, M. R. Duchen, M. F. Cordeiro, FASEB J. 28 (2014) 1317–1330. https://doi.org/10.1096/fj.13-235440
- F. Shakeel, S. Alshehri, M. Imran, N. Haq, A. Alanazi, Md. K. Anwer, Molecules 25 (2019) 171. https://doi.org/10.3390/molecules25010171
- D. De Ruysscher, L. Pang, C.-A. Mattelaer, M. Nautiyal, S. De Graef, J. Rozenski, S. V. Strelkov, E. Lescrinier, S. D. Weeks, A. Van Aerschot, Bioorg. Med. Chem. 28 (2020) 115580. . https://doi.org/10.1016/j.bmc.2020.115580
- M. S. Alwhibi, D. A. Soliman, H. al khaldy, A. Alonaizan, N. Abdulhaq Marraiki, M. El-Zaidy, M. S. AlSubeie, J. King Saud Univ. - Sci. 32 (2020) 3372–3379. https://doi.org/10.1016/j.jksus.2020.09.024
- M. A. Alshehri, J. K. Baskaradoss, A. Geevarghese, R. Ramakrishnaiah, D. N. Tatakis, Dent. Mater. J. 34 (2015) 148–153. https://doi.org/10.4012/dmj.2013-317
- A. S. Simbirtsev, V. G. Konusova, G. Sh. Mchelidze, E. Z. Fidarov, B. A. Paramonov, V. Yu. Chebotarev, Bull. Exp. Biol. Med. 133 (2002) 457–460. https://doi.org/10.1023/A:1019805603373
- E. Santovito, J. das Neves, D. Greco, V. D’Ascanio, B. Sarmento, A. F. Logrieco, G. Avantaggiato, Artif. Cells Nanomedicine Biotechnol. 46 (2018) 414–422. https://doi.org/10.1080/21691401.2018.1496924
- Y. Ito, T. Ito, K. Yamashiro, F. Mineshiba, K. Hirai, K. Omori, T. Yamamoto, S. Takashiba, Odontology 108 (2020) 57–65. https://doi.org/10.1007/s10266-019-00456-0
- T. A. Söderberg, R. Gref, S. Holm, T. Elmros, G. Hallmans, Scand. J. Plast. Reconstr. Surg. Hand Surg. 24 (1990) 199–205. https://doi.org/10.3109/02844319009041279
- C. Vilanova, M. Marín, J. Baixeras, A. Latorre, M. Porcar, PLoS ONE 9 (2014) e100740. https://doi.org/10.1371/journal.pone.0100740
- K. Sirivibulkovit, S. Nouanthavong, Y. Sameenoi, Anal. Sci. 34 (2018) 795–800. https://doi.org/10.2116/analsci.18P014
- S. R. Ahamad, A. R. Al-Ghadeer, R. Ali, W. Qamar, S. Aljarboa, Saudi Pharm. J. 25 (2017) 788–794. https://doi.org/10.1016/j.jsps.2016.10.011
- T. Liu, W. Wang, M. Liu, Y. Ma, F. Mu, X. Feng, Y. Zhang, C. Guo, Y. Ding, A. Wen, Int. Immunopharmacol. 89 (2020) 107094. https://doi.org/10.1016/j.intimp.2020.107094
- Y.-H. Liu, W.-L. Liang, C.-C. Lee, Y.-F. Tsai, W.-C. Hou, Food Chem. 129 (2011) 423–428. https://doi.org/10.1016/j.foodchem.2011.04.094
- V. Cuzzucoli Crucitti, L. M. Migneco, A. Piozzi, V. Taresco, M. Garnett, R. H. Argent, I. Francolini, Eur. J. Pharm. Biopharm. 125 (2018) 114–123. https://doi.org/10.1016/j.ejpb.2018.01.012
- A. Mirgorodskaya, R. Kushnazarova, R. Pavlov, F. Valeeva, O. Lenina, K. Bushmeleva, D. Kuryashov, A. Vyshtakalyuk, G. Gaynanova, K. Petrov, L. Zakharova, Molecules 27 (2022) 6447. https://doi.org/10.3390/molecules27196447
- C. M. Spagnol, R. P. Assis, I. L. Brunetti, V. L. B. Isaac, H. R. N. Salgado, M. A. Corrêa, Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 219 (2019) 358–366. https://doi.org/10.1016/j.saa.2019.04.025
- Y.-Z. Zheng, G. Deng, R. Guo, Z.-M. Fu, D.-F. Chen, Bioorganic Chem. 105 (2020) 104341. https://doi.org/10.1016/j.bioorg.2020.104341
- D. Chavarria, S. Benfeito, P. Soares, C. Lima, J. Garrido, P. Serrão, P. Soares-da-Silva, F. Remião, P. J. Oliveira, F. Borges, Eur. J. Med. Chem. 243 (2022) 114740. https://doi.org/10.1016/j.ejmech.2022.114740
- Z. Boual, G. Pierre, A. Kemassi, S. Mosbah, F. Benaoun, C. Delattre, P. Michaud, 27 (2020) 50-55.
- R. A. A. Eid, Saudi Dent. J. 33 (2021) 44–54. https://doi.org/10.1016/j.sdentj.2019.11.011
- X. Q. Li, Y. Chen, G. C. Dai, B. B. Zhou, X. N. Yan, R. X. Tan, J. Ethnopharmacol. 272 (2021) 113934. https://doi.org/10.1016/j.jep.2021.113934
- B. Ahmad, M. Batool, Q. ul Ain, M. S. Kim, S. Choi, Int. J. Mol. BOUAL, Zakaria, PIERRE, Guillaume, KEMASSI, Abdellah, et al. Chemical Composition and Biological Activities of Water-Soluble Polysaccharides from Commiphora Myrrha (Nees) Engl. GUM. Analele Universităţii din Oradea, Fascicula Biologie, 2020, vol. 27, no 1, p. 50-55.Sci. 22 (2021) 9124. https://doi.org/10.3390/ijms22179124
- F. Ibrahim, M. S. Elgawish, E. Mehana, S. M. El-Adl, M. M. Baraka, S. M. Ibrahim, M. M. Sebaiy, Chem. Res. Toxicol. 33 (2020) 2647–2658. https://doi.org/10.1021/acs.chemrestox.0c00285
- M. Lai, L. Zhang, L. Lei, S. Liu, T. Jia, M. Yi, Ind. Crops Prod. 144 (2020) 112065. https://doi.org/10.1016/j.indcrop.2019.112065
- M. G. de Lima Silva, L. Y. S. da Silva, T. S. de Freitas, J. E. Rocha, R. L. S. Pereira, S. R. Tintino, M. R. C. de Oliveira, A. O. B. P. Bezerra Martins, M. C. P. Lima, G. C. Alverni da Hora, C. L. G. Ramalho, H. D. M. Coutinho, I. R. A. de Menezes, Process Biochem. 122 (2022) 363–372. https://doi.org/10.1016/j.procbio.2022.10.010
- G. Sharma, S. Rao, A. Bansal, S. Dang, S. Gupta, R. Gabrani, Biologicals 42 (2014) 1–7. https://doi.org/10.1016/j.biologicals.2013.11.001
- Z. Pang, R. Raudonis, B. R. Glick, T.-J. Lin, Z. Cheng, Biotechnol. Adv. 37 (2019) 177–192. https://doi.org/10.1016/j.biotechadv.2018.11.013
- M. Alhejaili, D. W. Olson, C. Velázquez, M. Janes, C. Boeneke, K. J. Aryana, J. Dairy Sci. 102 (2019) 2011–2016. https://doi.org/10.3168/jds.2018-14831
- A. Daina, O. Michielin, V. Zoete, Sci. Rep. 7 (2017) 42717. https://doi.org/10.1038/srep42717
- M. Anza, M. Endale, L. Cardona, D. Cortes, R. Eswaramoorthy, J. Zueco, H. Rico, M. Trelis, B. Abarca, Adv. Appl. Bioinforma. Chem. Volume 14 (2021) 117–132. https://doi.org/10.2147/AABC.S323657
- E. Netto, R. Netto, M. Santana, J. Moura-Neto, L. Ferreira, Asian Pac. J. Cancer Prev. 22 (2021) 2289–2294. https://doi.org/10.31557/APJCP.2021.22.7.2289
- K. N. Theken, C. R. Lee, L. Gong, K. E. Caudle, C. M. Formea, A. Gaedigk, T. E. Klein, J. https://doi.org/10.1002/cpt.1830 A. G. Agúndez, T. Grosser, Clin. Pharmacol. Ther. 108 (2020) 191–200.
- V. Chubukov, F. Mingardon, W. Schackwitz, E. E. K. Baidoo, J. Alonso-Gutierrez, Q. Hu, T. S. Lee, J. D. Keasling, A. Mukhopadhyay, Appl. Environ. Microbiol. 81 (2015) 4690–4696. https://doi.org/10.1128/AEM.01102-15
- Q. Wei, K. Harada, S. Ohmori, K. Minamoto, C. Wei, A. Ueda, J. Occup. Health 48 (2006) 480–486. https://doi.org/10.1539/joh.48.480
- S. G. Kshirsagar, R. V. Rao, Medicina (Mex.) 57 (2021) 217. https://doi.org/10.3390/medicina57030217


