
- Keywords: Metallic trace elements, adsorption, desorption, Elovich model, Langmuir model, Freundlich model
Copyright (c) 2024 JCChemS

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
ABSTRACT
The study of the processes of adsorption – desorption of metallic trace elements or heavy metals in soils is crucial for the proposal of effective strategies for mitigation, reduction or elimination of these in natural systems.
This work presents a study of the processes of adsorption and/desorption of Cu, Mn, Pb and Zn in two Chilean soils: Alhué (VI Region, Chile) and Nueva Braunau (X Region, Chile), both of volcanic origin and agricultural use.
The soil samples studied were dried and sieved (< 2 mm or < 0.63 mm). The physicochemical characterization: pH, CE, MO, COT, N, P, CIC and texture was performed using described methods. The total fraction of each metal was obtained by microwave digestion with HNO3 and HCl. The contact time needed to reach equilibrium was determined by leaving the soil with the metal solution at different times (3, 6, 12, 24, 48 and 72 hours). The adsorption process was performed by a batch experiment, mixing different aliquots of metal solution with balancing solution (KNO3) 0.1 molL-1. Desorption was made from the 50/50 metal/balancing solution. The metals in the different soil samples and the total fraction in the soil were determined by AAS.
The distribution of the total fraction of metals in decreasing order is in Alhué soil: Mn > Pb > Cu > Zn and in Nueva Braunau soil: Mn > Pb > Zn > Cu. It was determined that the contact time at which metals reach equilibrium is 48 hours for both soils, fitting appropriately with Elovich's model.
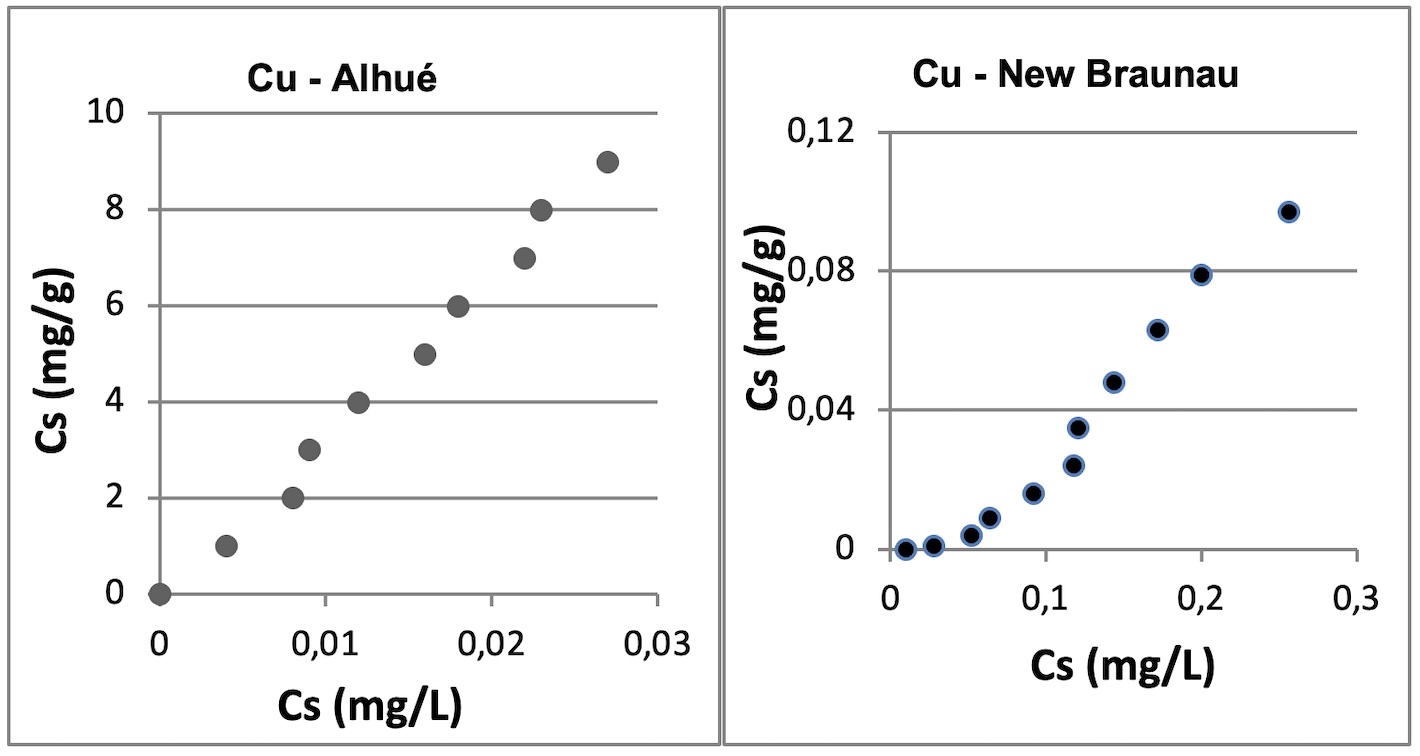
The decreasing order of adsorption in the Alhué soil is: Cu > Zn > Pb > Mn and in the Nueva Braunau soil: Cu > Pb > Zn > Mn.
The determination of the type of adsorption was made through the application of adsorption models of Langmuir and Freundlich. Cu adjusted according to the Langmuir model on both soils. Cu and Zn conformed to Freunlich's model.
The desorption results are: Alhué soil: Pb > Mn > Zn > Cu and Nueva Braunau soil: Mn > Pb > Zn > Cu.
The negative ΔG° (Gibbs free energy change) values obtained in the presents study indicate that the adsorption of metals onto soil samples is a spontaneous process and that the adsorption was an exothermic process excep for Mn in both soils.
References
- REFERENCES
- Vidal, M.; Santos, M.J.; Abrão, T.; Rodríguez, J.; Rigol, A., Geoderma, 149, 189, (2009).
- Elbana, T.A.; Selim, H.M., Geoderma, 338, 78, (2019).
- Bradl, H.B., J. Colloid Interface Sci., 277, 1, (2004).
- Selim, H.M.; Zhang, H., J. Environ. Qual., 42, 640, (2013).
- Ming, H.; Naidu, R.; Sarkar, B.; Lamb, D.T.; Liu, Y.; Megharaj, M.; Sparks, D., Geoderma., 268, 60, (2016).
- Wu, Z.; Chen, Y.; Han, Y.; Ke, T.; Liu, Y., Sci. Total Environ., 717, 137212, (2020).
- Huang, B.; Li, Z.; Huang, J.; Guo, L.; Nie, X.; Wang, Y.; Zhang, Y.; Zeng, G., J. Hazard. Mater., 264, 176, 014).
- Fernández-Calviño, D.; Pérez-Novo, C.; Bermúdez-Couso, A.; López-Periago, E.; Arias-Estévez, M., Geoderma, 159, 417, (2010).
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.G.; Pigna, M., J. Soil Sci. Plant Nutr., 10, 268, (2010).
- Dini´c, Z.; Maksimovi´c, J.; Stanojkovi´c-Sebi´c, A.; Pivi´c, R., Agronomy, 9, 856 (2019).
- Huang, Y.; Fu, C.; Fang, F.; Ouyang, W.; Gou, J. E, Ecotoxico. Environ. Saf., 185, 109695, (2019).
- Abdin, Y.; Usman, A.; Ok, Y.S.; Tsnag, Y.F.; Al-Wabel, M., Environ. Res., 181, 108846, (2020).
- Sparks, D.L. Environmental Soil Chemistry, 2nd ed.; Academic Press: San Diego, CA, USA, p. 352, (2003).
- Antoniadis, V.; Tsadilas, C.D., Appl.Geochem., 22, 2375, (2007).
- Silva-Yumi, J.; Escudey, M.; Gacitua, M.; Pizarro, C., Geoderma, 319, 70, (2018).
- Sipos, P.; Tóth, A.; Kis, V.K.; Balázs, R.; Kovács, I.; Németh, T., J. Soils Sedim., 19, 1775, (2019).
- Alloway, B.J., 1995b. Chapter 2: Soil process and the behavior of the heavy metals. In: Alloway, B.J. (ed). Heavy metals in soils. Blackie Academic and Professional, London, 2nd edition. 179-205.
- Colombo L.D., Mangione, S.B., Figlioglia A., Agr. Med. Inter. J. of Agric. Sci. 128, 273, (1998).
- Baird C., 1999. Environmental Chemistry. 2nd Ed. W.H. Freman & Company.
- Moolenaar, S.W., Lexmond, T.M., Van der Zee, S.E.A.T.M., Agriculture, Ecosystems and Evironment, 66, 71, (1997).
- Gulson B.L., Mizon K.J., Korsch M.J., Howarth D., The science of the total environment., 181, 223, (1996).
- Rieuwerts, J.S., Thornton, L., Farago, M. E., Ashmore, M. R., Chemical Speciation and Bioavailability, 10, 61, (1998).
- Giller, K., Witter, E., Mcgrath, S.P., Soil Biol. Biochem. 30, 564, (1998).
- Gjoka, F., Felix-Henningsen, P., Wegener, I., Salillari, I., Beqiraj, A., Enviromental Monitoring and Assessment. 172, 517, (2010).
- Castillo, M., Ortega, E., Rubí, M., Edafología 9, 295, (2002).
- Fassbender, H. W. y Bornemisza, E., Fenómenos de adsorción y cambios en suelos. En: Química de Suelos con énfasis en suelos de América Latina. Instituto Interamericano de Cooperación para la Agricultura. San José. Costa Rica. Servicio Editorial IICA. 137, (1987).
- Atkins, P.W., Química Física, 6ta edición. 28 (28.3), 862 (1999).
- Rodriguez, R., Linares, R., Guadalupe, E., Revista del Instituto de Investigaciones FIGMMG. 12 (Nº 24), 108-117. UNMSM (2009).
- Kendorf, H., Schnitzer, M., Geochim. Cosmochim. Acta, 44, 1701, (1980).
- Copaja S. V; Gatica-Jeria, P., J. Chil. Chem. Soc., 66, N°1 (2021)
- Copaja S. V.; Sepúlveda C., J. Chil. Chem. Soc., 67, N°3 (2022)
- Gomes, P.C., Fontes, M.P.F., da Silva, D.G., Mendoca, E., Netto, A.R., Soil Sci. Am. J. 65, 1115, (2001).
- Vega, F.A., Covelo, E.F., Andrade, M.L., Spanish Journal of Soil Science (SJSS)
- (2001).
- Tessier, A., Carignan, R., Dubreuil, B., Rapin, F., Cosmochim. , 53, 1511, (1989).
- Kooner, Z., Environ Geol., 21, 242, (1993).
- Turner, N. H., Journal of Catalysis, 36, 262, (1975).
- Dali-youcef, N., Ouddanea, B., Derriche, Z., 2006. Journal of Hazardous Materials, 137, 1263, (2006).
- Chamorro, A.F., Sanchez, R.D., Revista de ciencias 16, 145, (2012).
- OECD. Guidelines for testing of chemicals, Section 1 (106): Adsorption-Desorption using batch equilibrium method in soils. Environmental Health and Safety Division, Organisation for Economic Co-operation and Development (OECD), Environment. Directorate, Paris, France. 2000
- Copaja, S. V. Bravo, H. R., Muñoz P., J. Chil. Chem. Soc., 57, 1091, (2012)
- Copaja S. V., Mauro L.., Vega-Retter, C., Véliz, D., J. Chil. Chem. Soc., 65, 4778, (2020).
- Sadzawka, M.A, Carrasco, M., Grez, R., Mora, M., Flores, H., Neaman, A., 2006. Métodos de análisis de suelos. Instituto de investigación Agropecuarias, serie la platina (N°16), pag. 17-18, 19-20, 23-27, 43-56, 95-96, 111.
- L. C. Blackemore, P. L. Scarle, B. K. Daly, B. K. In Methods for Chemical of Analysis of Soils N. Z. Soil Bureau Scientific Report, 1987; pp 18-34
- Bray, R.H. and Kurtz, L.T., Soil Science 59, 39, (1945).
- EPA Method 3051, Microwave assisted acid digestion of sediments, sludges, soils, and oils, (1994).
- Feng-Chin Wu, Ru-Ling Tseng, Ruey-Shin Juang. Chemical Engineering Journal, 150, 366, (2009).
- Chen, X., Information 6, 14, (2015).
- M. R. Shariff, International Journal of Engineering Research and Development 1, 55, (2012).
- K. S. Ahmad, N. Rashid, M. F. Nazar, S. Tazaiyen, Journal of the Chemical
- Society of Pakistan , 34-35, 1017, (2014).
- I. Langmuir, J. Am. Chem. Soc. 38, 2221–2295 (1916).
- A. O. Dada, A. P. Olalekan, A. M. Olatunya, O. Dada, Journal of AppliedChemistry, 3, 38, (2012).
- J. Porta, M. Lopez – Acevedo, R. Porch, Edafología: uso y protección de
- suelos. 3ed. 272, 2014.
- Y. Liu, Z. Xu, X. Wu, W. Gui, G. Zhu., Journal of Hazardous Materials.
- , 462, (2010).
- Schlatter, J., Grez, R., Gerding, V. Manual para reconocimiento de Suelos, Instituto de Silvicultura – Facultad de Ciencias Forestales. Universidad Austral de Chile. 53.
- Lasat, M., 2001.The uses of plants for removal of toxic metal from contaminated soil. USEPA
- Silviera, M.L.A., Alleonl, L.R.F., Ghilherme, L.R.G., Scientia Agricola 60 (4), 793, (2003).
- Honorato, R.I., 2000. Manual de Edafología, cuarta edición, Ediciones Universidad Católica de Chile, 81, 82, (2002).
- Keller, C., Domergue, F.L., Vedy, L.C., 1992. Chapter.4: Soil interaction. In: Vernet, J.P., Impact of heavy metals on the environment. 2: Heavy metals in the environment. Editorial Elsevier, New York. 247-269, (1992).
- Acevedo, E.; Carrasco, M.; León, O.; Martínez, E.; Silva, P. Criterios de calidad de suelo agrícola. Ministerio de Agricultura. Gobierno de Chile. 205 p. (2005).
- Giles, C., Mac Ewan, T., Nakhwa, S. and Smith, D., J. Chem. Soc. 111, 3973, (1960).
- Prasanna, Y., Kumar, P. King, V.S.R.K. Prasad. 2010. “Comparison for adsorption modelling of cooper and zinc from aqueous solution by Ulvafasciatasp”. Journal of Hazardous Materials B137, 1246, (2010).
- Gamze, N., Elevli, S., Mesci, B., 2011. Adsorption of copper and zinc ions on illite: Determination of the optimal conditions by the statistical design of experiments. Clay Science 52, 392-399.
- Besoain E., 1985. Los Suelos. En: Suelos Volcánicos de Chile. Primera Edición (ed. J. Tosso), Instituto de Investigación Agropecuaria (INIA), Ministerio de Agricultura. Santiago, Chile. 1, 24-27, 29.
- Conti, M., 2009. Principios de edafología, con énfasis en suelos argentinos. Editorial Facultad Agronomía, Segunda Edición.

