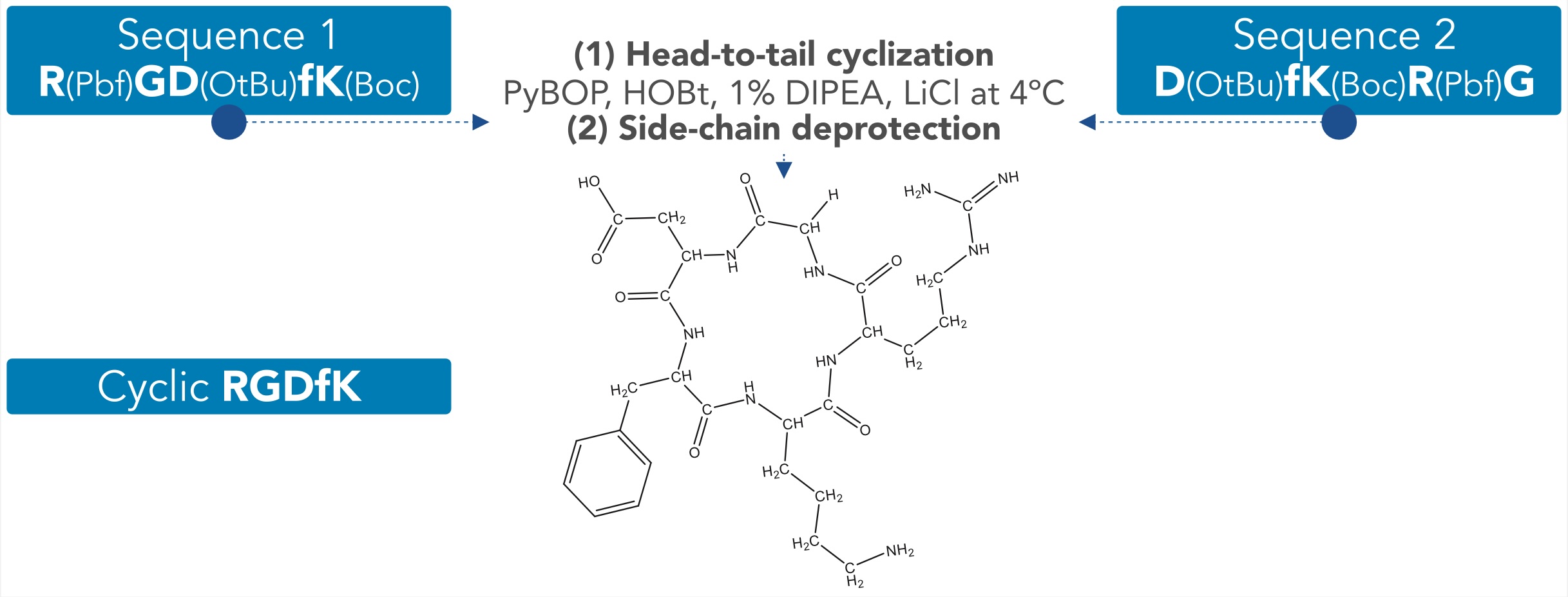
Improvement of Head-to-Tail RGD peptide Cyclization Efficiency Combining Low Temperature and Lithium Chloride

- Cyclic(RGDfK),
- head-to-tail cyclization,
- Integrins,
- peptide cyclization conditions,
- Targeting
Copyright (c) 2024 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
RGD-containing peptides (linear and cyclic) have been developed to target some types of cancer cells through interaction with the αvβ3 Integrin. Due to their reduced conformational freedom, cyclic peptides exhibit improved metabolic stability and binding affinity/specificity to their molecular targets. However, macrocyclization is considered a significant synthetic challenge that is affected by the ring size, peptide sequence, and reaction conditions, making tetra- and pentapeptides more difficult to cyclize. In this work, we report some optimized cyclization conditions for the obtention of a cyclic RGDfK peptide that combine the use of low reaction temperature and the addition of LiCl, a combination not yet reported that resulted in a reduction of the formation of oligomers and an improvement of the cyclization efficiency.
References
- S.H. Joo. Biomol. Ther. 20, 19–26, (2012)
- M.E.G Moral, T.J. Siahaan. Curr Top. Med. Chem. 17(32), 3425-3443, (2017)
- X. Dou, T. Nomoto, H. Takemoto. Sci. Rep. 8, 8126, (2018)
- L. Zhao, X. Wen, W. Xu. J. Nucl. Med. 64(8), 1210-1217, (2023)
- H.J. Kim, S.J. You, D.H. Yang. Biomater. Sci. 8(15), 4334-4345 (2020)
- S.J. Bogdanowich-Knipp, S. Chakrabarti, T.D. Williams. J. Pept. Res. 53(5), 530-41 (1999)
- D. Lobeek, M. Rijpkema, S. Terry. Eur. J. Nucl. Med. Mol. Imaging. 47(11), 2647-2655 (2020)
- F. Debordeaux, L. Chansel-Debordeaux, J.B. Pinaquy. Nucl. Med. Biol. 62, 31-46, (2018)
- T.M. Cheng, W.J. Chang, H.Y. Chu. Cells. 10(7), 1684, (2121)
- S. Fu, X. Xu, Y. Ma. J. Drug. Target. 27(1), 1-11, (2019)
- J. Liu, X. Cheng, X. Tian. ACS. Med. Chem. Lett. 29(7), 896-900, (2019)
- K. Ahmad, E.J. Lee, S. Shaikh. Semin. Cancer. Biol. 69, 325-336 (2121)
- N. Li, S. Qiu, Y. Fang. Biology. 10(7), 688, (2121)
- M. Paradís-Bas, J. Tulla-Puche, F. Albericio. Chem. Soc. Rev. 7:45(3), 631-54, (2016)
- A. Thakkar, T.B. Trinh, D. Pei. ACS. Comb. Sci. 11;15(2), 120-9 (2013)
- J. Tang, Y. He, H. Chen. Chem. Sci. 8(6), 4565-4570, (2017)
- A. El-Faham, F. Albericio. Chem. Rev. 111, 6557-602, (2021)
- F.A. Robey. J. Pept. Res. 56(3), 115-20, (2000)
- A. Thaler, D. Seebach, F. Cardinaux. Helv. Chim. Acta. 74, 628–643, (1991)
- K. Arnold, B. Davied, R.L. Giles. Adv. Synth. Catal. 348, 813-820, (2006)
- F. Guzmán, A. Mónica, R. Tanya. Electron. J. Biotechnol. 64, 27-33 (2023)
- J.S. Davies. J. Pept. Sci. 9(8), 471-501, (2003)
- V. Martí-Centelles, M.D. Pandey, M. Burguete. Chem. Rev. 115(16), 8736-834 (2015)

