WORKFLOW FOR BIO-GUIDED FRACTIONATION OF ANTIMICROBIAL POLYPHENOLS FROM UGNI MOLINAE LEAVES USING MIRCOFRACTIONATION AND CENTRIFUGAL PARTITION CHROMATOGRAPHY: IN VITRO AND IN SILICO STUDIES
- Ugni molinae, Centrifugal Partition Chromatography, Jack Bean urease, Helicobacter pylori, Microscale Thermophoresis, molecular docking
Copyright (c) 2024 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
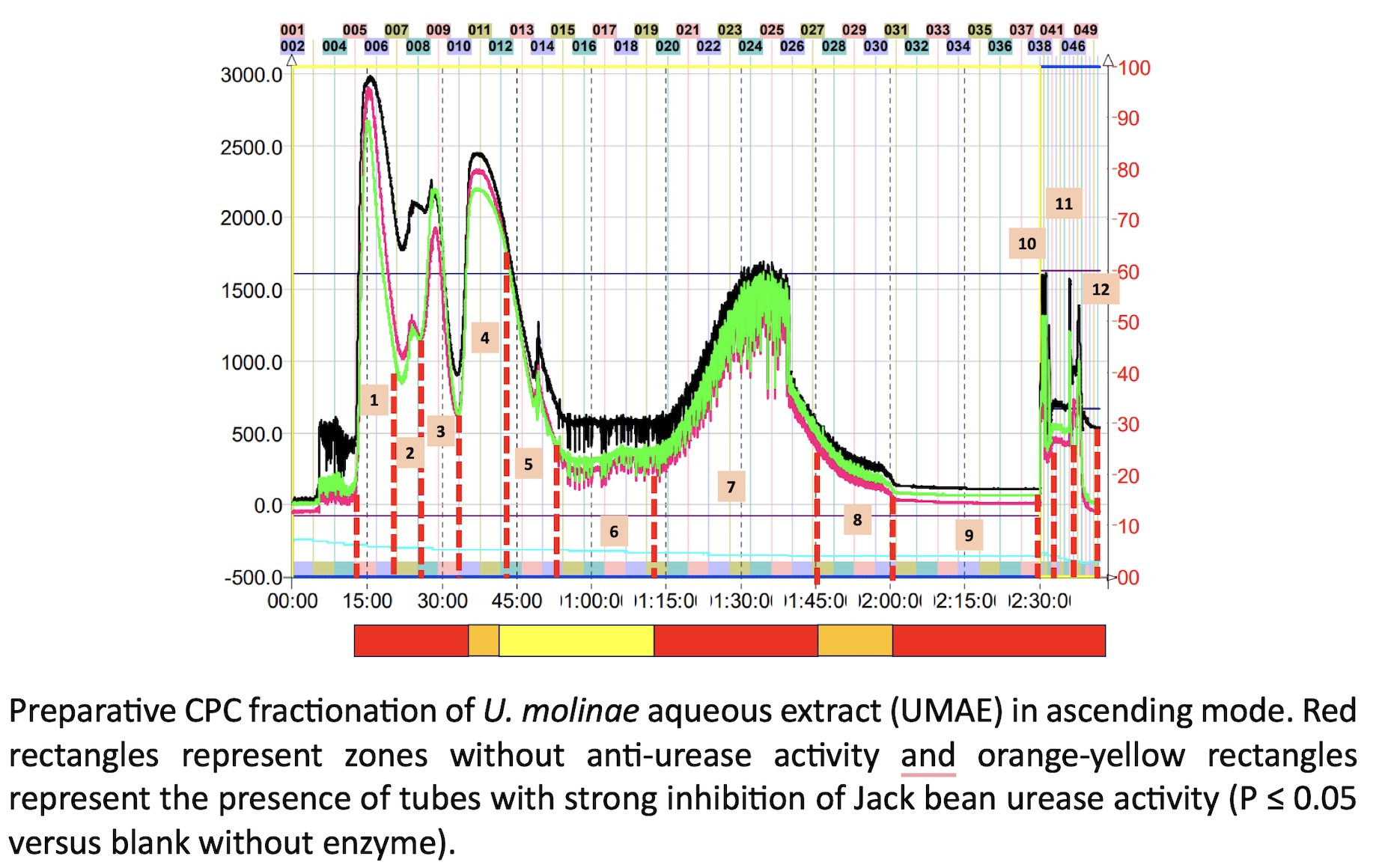
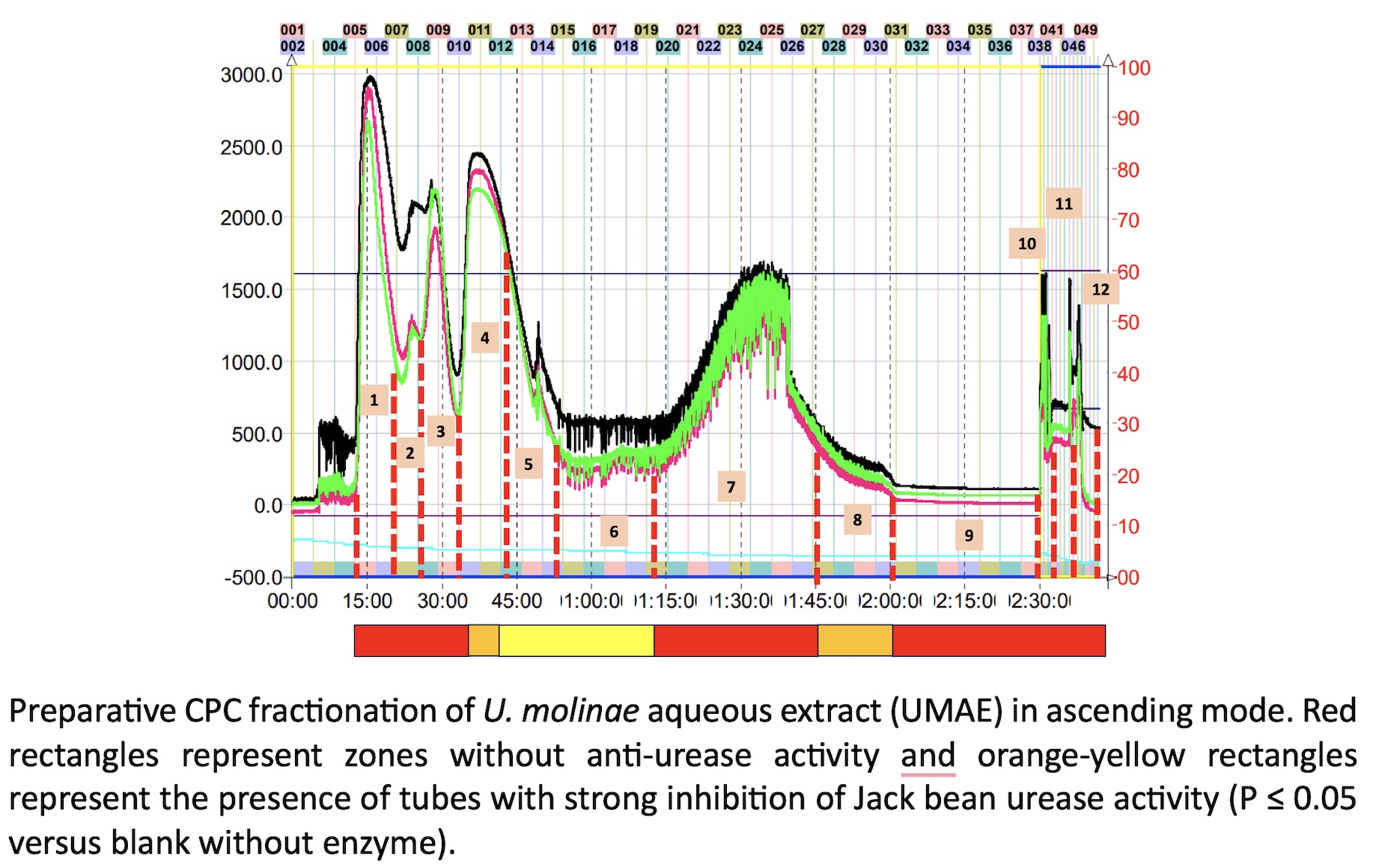
The misuse of antibiotics has led to high levels of drug-resistance in specific pathogens, promoting the search of molecules from different natural sources or the design of novel drug candidates. Medicinal and edible plants are a rich source of bioactive compounds, in particular those of polyphenol class. In the present work, we screen the antimicrobial properties of an aqueous extract prepared from the leaves of Ugni molinae (Turz) against a panel of pathogenic bacteria strains formed by Helicobacter pylori (ATCC 43504), Listeria monocytogenes (ATCC 7644), Staphylococcus aureus (ATCC 9144), Escherichia coli (ATCC 11775), and Salmonella enterica (ATCC 13076). Preliminary fast HPLC-micro fractionation allows the identification of potential urease inhibitors using high throughput urea-phenol microplate assay. Afterwards, preparative fractionation by Centrifugal Partition Chromatography (CPC) allow to select the specific bioactive fractions. A combination of antimicrobial tests, enzyme assays and molecular docking resulted in the identification by HPLC-MS/MS of two quercetin-O-(6´´-O-galloyl)-hexosides as the most dominant compounds in the active CPC-fractions. These bioactive compounds were quercetin-3-O-(6´´-O-galloyl)-b-galactopyranoside (hyperin 6”-gallate) and quercetin-3-O- b -D-(6´´-O-galloyl)- b -glucopyranoside (tellimoside). The molecular docking evaluation revealed that hyperin-6´´-gallate enter the binding site of urease and bind in through pi-cation, pi–pi, and H-bond interactions. In concordance with the in-silico assay, the CPC fraction containing this compound has the lowest values of IC50 for Jack bean (0.41 ± 0.08 µg/mL) and Helicobacter pylori (0.28 ± 0.08 µg/mL) ureases, respectively. Moreover, Label-Free Microscale Thermophoresis (MST) analysis suggest that this flavonoid forms a complex with urease, even inducing protein aggregation. In conclusion, Ugni molinae leaves has potent anti-urease flavonoids that can contribute to significantly reduce the acclimation of H. pylori in the acidic environmental of gastric mucosa.
References
- López, J.; Vega-Gálvez, A.; Rodríguez, A.; Uribe, E.; Bilbao-Sainz, C. Review MURTA (Ugni Molinae Turcz.): A Review on Chemical Composition, Functional Components and Biological Activities of Leaves and Fruits. Chilean J. Agric. Anim. Sci., ex Agro-Ciencia 2018, 34, 43–56.
- Schreckinger, M.E.; Lotton, J.; Lila, M.A.; de Mejia, E.G. Berries from South America: A Comprehensive Review on Chemistry, Health Potential, and Commercialization. J Med Food 2010, 13, 233–246, doi:10.1089/jmf.2009.0233.
- Alfaro, S.; Mutis, A.; Palma, R.; Quiroz, A.; Seguel, I.; Scheuermann, E. Influence of Genotype and Harvest Year on Polyphenol Content and Antioxidant Activity in Murtilla (Ugni Molinae Turcz) Fruit. J Soil Sci Plant Nutr 2013, 0–0, doi:10.4067/S0718-95162013005000007.
- Peña-Cerda, M.; Arancibia-Radich, J.; Valenzuela-Bustamante, P.; Pérez-Arancibia, R.; Barriga, A.; Seguel, I.; García, L.; Delporte, C. Phenolic Composition and Antioxidant Capacity of Ugni Molinae Turcz. Leaves of Different Genotypes. Food Chem 2017, 215, 219–227, doi:10.1016/j.foodchem.2016.07.159.
- Avello Lorca, M.; Pastene Navarrete, E.; Barriga, A.; Bittner Berner, M.; Ruiz Ponce, E.; Becerra Allende, J. Chemical Properties and Assessment of the Antioxidant Capacity of Native Species from the Genus Ugni. Revista Cubana de Plantas Medicinales 2016, 21.
- Avello Lorca, M.; Pastene Navarrete, E.; González Riquelme, M.; Bittner Berner, M.; Becerra Allende, J. In Vitro Determination of the Antioxidant Capacity of Extracts and Phenolic Compounds from Ugni Molinae Turcz. Leaves. Revista Cubana de Plantas Medicinales 2013, 18.
- Avello, M.; Torres, E.; Carvajal, R.I.; Pastene, E. Effect of in Vitro Digestion Gastrointestinal of the Extract Aqueou of Leaves of Ugni Molinae, on the Viability of Colorectal Cancer Cells. Journal of the Chilean Chemical Society 2021, 66, doi:10.4067/S0717-97072021000305268.
- Torres Vallejos, E.; Avello, M.; Pastene, E. Identification of Water-Soluble Compounds Contained in Aqueos Extracts and Fractions Obtained from Leaves of Ugni Molinae to Determine Their Effect on the Viability of Humna Gastric Cancer Cells. Journal of the Chilean Chemical Society; Vol 65 No 2 (2020): Journal of the Chilean Chemical Society; 4849-4852 2020.
- Jara-Moreno, D.; Riveros, A.L.; Barriga, A.; Kogan, M.J.; Delporte, C. Inhibition of β-Amyloid Aggregation of Ugni Molinae Extracts. Curr Pharm Des 2020, 26, 1365–1376, doi:10.2174/1381612826666200113160840.
- Pérez-Arancibia, R.; Ordoñez, J.L.; Rivas, A.; Pihán, P.; Sagredo, A.; Ahumada, U.; Barriga, A.; Seguel, I.; Cárdenas, C.; Vidal, R.L.; et al. A Phenolic-Rich Extract from Ugni Molinae Berries Reduces Abnormal Protein Aggregation in a Cellular Model of Huntington’s Disease. PLoS One 2021, 16, e0254834, doi:10.1371/journal.pone.0254834.
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, Vol. 10, Page 188 2021, 10, 188, doi:10.3390/ANTIOX10020188.
- Piwowarski, J.P.; Granica, S.; Stefańska, J.; Kiss, A.K. Differences in Metabolism of Ellagitannins by Human Gut Microbiota Ex Vivo Cultures. J Nat Prod 2016, 79, 3022–3030, doi:10.1021/acs.jnatprod.6b00602.
- Luo, C.; Wei, X.; Song, J.; Xu, X.; Huang, H.; Fan, S.; Zhang, D.; Han, L.; Lin, J. Interactions between Gut Microbiota and Polyphenols: New Insights into the Treatment of Fatigue. Molecules 2022, 27.
- Cires, M.J.; Wong, X.; Carrasco-Pozo, C.; Gotteland, M. The Gastrointestinal Tract as a Key Target Organ for the Health-Promoting Effects of Dietary Proanthocyanidins. Front Nutr 2017, 3, 1.
- Andriamihaja, M.; Lan, A.; Beaumont, M.; Grauso, M.; Gotteland, M.; Pastene, E.; Cires, M.J.; Carrasco-Pozo, C.; Tomé, D.; Blachier, F. Proanthocyanidin-Containing Polyphenol Extracts from Fruits Prevent the Inhibitory Effect of Hydrogen Sulfide on Human Colonocyte Oxygen Consumption. Amino Acids 2018, 50, doi:10.1007/s00726-018-2558-y.
- Cires, M.J.; Navarrete, P.; Pastene, E.; Carrasco-Pozo, C.; Valenzuela, R.; Medina, D.A.; Andriamihaja, M.; Beaumont, M.; Blachier, F.; Gotteland, M. Protective Effect of an Avocado Peel Polyphenolic Extract Rich in Proanthocyanidins on the Alterations of Colonic Homeostasis Induced by a High-Protein Diet. J Agric Food Chem 2019, 67, 11616–11626, doi:10.1021/acs.jafc.9b03905.
- Cires, M.J.; Navarrete, P.; Pastene, E.; Carrasco-Pozo, C.; Valenzuela, R.; Medina, D.A.; Andriamihaja, M.; Beaumont, M.; Blachier, F.; Gotteland, M. Effect of a Proanthocyanidin-Rich Polyphenol Extract from Avocado on the Production of Amino Acid-Derived Bacterial Metabolites and the Microbiota Composition in Rats Fed a High-Protein Diet. Food Funct 2019, 10, 4022–4035, doi:10.1039/c9fo00700h.
- Wong, X.; Carrasco-Pozo, C.; Escobar, E.; Navarrete, P.; Blachier, F.; Andriamihaja, M.; Lan, A.; Tomé, D.; Cires, M.J.; Pastene, E.; et al. Deleterious Effect of P-Cresol on Human Colonic Epithelial Cells Prevented by Proanthocyanidin-Containing Polyphenol Extracts from Fruits and Proanthocyanidin Bacterial Metabolites. J Agric Food Chem 2016, 64, 3574–3583, doi:10.1021/acs.jafc.6b00656.
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11.
- Medicinales Aromáticas, P. Boletín Latinoamericano y Del Caribe De. 2009.
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter Pylori Infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30.
- Kusters, J.G.; Kuipers, E.J. Antibiotic Resistance of Helicobacter Pylori. J Appl Microbiol 2001, 90, 134S-144S, doi:10.1046/j.1365-2672.2001.01362.x.
- Sachs, G.; Scott, D.R. Helicobacter Pylori: Eradication or Preservation. F1000 Med Rep 2012, 4, 7, doi:10.3410/M4-7.
- Mobley, H.L.T.; Hu, L.T.; Foxall, P.A. Helicobacter Pylori Urease: Properties and Role in Pathogenesis. Scand J Gastroenterol 1991, 26, 39–46, doi:10.3109/00365529109098223.
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter Pylori Infection. Nat Rev Dis Primers 2023, 9, doi:10.1038/s41572-023-00431-8.
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter Pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin Microbiol Rev 2010, 23, 713–739.
- Ferreccio, C.; Rollán, A.; Harris, P.R.; Serrano, C.; Gederlini, A.; Margozzini, P.; Gonzalez, C.; Aguilera, X.; Venegas, A.; Jara, A. Gastric Cancer Is Related to Early Helicobacter Pylori Infection in a High-Prevalence Country. Cancer Epidemiology Biomarkers and Prevention 2007, 16, 662–667, doi:10.1158/1055-9965.EPI-06-0514.
- Roesler, B.M.; Botelho Costa, S.C.; Murilo, J.; Zeitune, R. Eradication Treatment of Helicobacter Pylori Infection: Its Importance and Possible Relationship in Preventing the Development of Gastric Cancer. International Scholarly Research Network ISRN Gastroenterology 2012, 2012, doi:10.5402/2012/935410.
- Ambrosi, C.; Sarshar, M.; Aprea, M.R.; Pompilio, A.; Di Bonaventura, G.; Strati, F.; Pronio, A.; Nicoletti, M.; Zagaglia, C.; Palamara, A.T.; et al. Colonic Adenoma-Associated Escherichia Coli Express Specific Phenotypes. Microbes Infect 2019, 21, 305–312, doi:10.1016/j.micinf.2019.02.001.
- Williams, M.A.; Schmidt, R.L.; Lenz, L.L. Early Events Regulating Immunity and Pathogenesis during Listeria Monocytogenes Infection. Trends Immunol 2012, 33, 488–495, doi:10.1016/j.it.2012.04.007.
- Flickinger, J.C.; Rodeck, U.; Snook, A.E. Listeria Monocytogenes as a Vector for Cancer Immunotherapy: Current Understanding and Progress. Vaccines (Basel) 2018, 6.
- Castro-Seriche, S.; Jerez-Morales, A.; Smith, C.T.; Sánchez-Alonzo, K.; García-Cancino, A. Candida Albicans, a Reservoir of Listeria Monocytogenes? Infection, Genetics and Evolution 2021, 90, 104779, doi:10.1016/j.meegid.2021.104779.
- Santativongchai, P.; Tulayakul, P.; Jeon, B. Enhancement of the Antibiofilm Activity of Nisin against Listeria Monocytogenes Using Food Plant Extracts. Pathogens 2023, 12, doi:10.3390/pathogens12030444.
- Liu, F.; Rajabi, S.; Shi, C.; Afifirad, G.; Omidi, N.; Kouhsari, E.; Khoshnood, S.; Azizian, K. Antibacterial Activity of Recently Approved Antibiotics against Methicillin-Resistant Staphylococcus Aureus (MRSA) Strains: A Systematic Review and Meta-Analysis. Ann Clin Microbiol Antimicrob 2022, 21, 37, doi:10.1186/s12941-022-00529-z.
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic Strategies To Counteract Antibiotic Resistance in MRSA Biofilm‐Associated Infections. ChemMedChem 2021, 16, 65–80, doi:10.1002/cmdc.202000677.
- Ejaz, M.; Syed, M.A.; Jackson, C.R.; Sharif, M.; Faryal, R. Epidemiology of Staphylococcus Aureus Non-Susceptible to Vancomycin in South Asia. Antibiotics 2023, 12, 972, doi:10.3390/antibiotics12060972.
- Jajere, S.M. A Review of Salmonella Enterica with Particular Focus on the Pathogenicity and Virulence Factors, Host Specificity and Antimicrobial Resistance Including Multidrug Resistance. Vet World 2019, 12, 504–521, doi:10.14202/vetworld.2019.504-521.
- Hendriksen, S.W.M.; Orsel, K.; Wagenaar, J.A.; Miko, A.; van Duijkeren, E. Animal-to-Human Transmission of Salmonella Typhimurium DT104A Variant. Emerg Infect Dis 2004, 10, 2225–2227, doi:10.3201/eid1012.040286.
- Hebel-Gerber, S.; García-Cancino, A.; Urbina, A.; Simirgiotis, M.J.; Echeverría, J.; Bustamante-Salazar, L.; Sáez-Carrillo, K.; Alarcón, J.; Pastene-Navarrete, E. Chilean Rhubarb, Gunnera Tinctoria (Molina) Mirb. (Gunneraceae): UHPLC-ESI-Orbitrap-MS Profiling of Aqueous Extract and Its Anti-Helicobacter Pylori Activity. Front Pharmacol 2021, 11, doi:10.3389/fphar.2020.583961.
- Chávez, F.; Aranda, M.; García, A.; Pastene, E. Antioxidant Polyphenols Extracted from Avocado Epicarp (Persea Americana Var. Hass) Inhibit Helicobacter Pylori Urease. Bol Latinoam Caribe Plantas Med Aromat 2011, 10.
- Pastene, E.; Troncoso, M.; Figueroa, G.; Alarcón, J.; Speisky, H. Association between Polymerization Degree of Apple Peel Polyphenols and Inhibition of Helicobacter Pylori Urease. J Agric Food Chem 2009, 57, doi:10.1021/jf8025698.
- Pastene, E.; Parada, V.; Avello, M.; Ruiz, A.; García, A. Catechin-Based Procyanidins from Peumus Boldus Mol. Aqueous Extract Inhibit Helicobacter Pylori Urease and Adherence to Adenocarcinoma Gastric Cells. Phytotherapy Research 2014, 28, 1637–1645, doi:10.1002/ptr.5176.
- Linke, P.; Amaning, K.; Maschberger, M.; Vallee, F.; Steier, V.; Baaske, P.; Duhr, S.; Breitsprecher, D.; Rak, A. An Automated Microscale Thermophoresis Screening Approach for Fragment-Based Lead Discovery. J Biomol Screen 2016, 21, 414–421, doi:10.1177/1087057115618347.
- Ribeiro, M.L.; Gerrits, M.M.; Benvengo, Y.H.B.; Berning, M.; Godoy, A.P.O.; Kuipers, E.J.; Mendonça, S.; Van Vliet, A.H.M.; Pedrazzoli, J.; Kusters, J.G. Detection of High-Level Tetracycline Resistance in Clinical Isolates of Helicobacter Pylori Using PCR-RFLP. FEMS Immunol Med Microbiol 2004, 40, 57–61, doi:10.1016/S0928-8244(03)00277-3.
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb Perspect Med 2016, 6, doi:10.1101/cshperspect.a025387.
- Mroczek, T.; Dymek, A.; Widelski, J.; Wojtanowski, K.K. The Bioassay-Guided Fractionation and Identification of Potent Acetylcholinesterase Inhibitors from Narcissus c.v. ‘Hawera’ Using Optimized Vacuum Liquid Chromatography, High Resolution Mass Spectrometry and Bioautography. Metabolites 2020, 10, 395, doi:10.3390/metabo10100395.
- McCallum, J.L.; Vacon, J.N.D.; Kirby, C.W. Ultra-Micro-Scale-Fractionation (UMSF) as a Powerful Tool for Bioactive Molecules Discovery. Molecules 2020, 25, 3677, doi:10.3390/molecules25163677.
- Wang, R.; Liu, Y.; Zhou, H.; Chen, Y.; Wang, J.; Zhang, X.; Yu, R.; Liang, X. Integration of Micro-Fractionation, High-Performance Liquid Chromatography-Ultraviolet Detector-Charged Aerosol Detector-Mass Spectrometry Analysis and Cellular Dynamic Mass Redistribution Assay to Accelerate Alkaloid Drug Discovery. J Chromatogr A 2020, 1616, 460779, doi:10.1016/j.chroma.2019.460779.
- Krajewska, B.; Van Eldik, R.; Brindell, M. Temperature- and Pressure-Dependent Stopped-Flow Kinetic Studies of Jack Bean Urease. Implications for the Catalytic Mechanism. In Proceedings of the Journal of Biological Inorganic Chemistry; October 2012; Vol. 17, pp. 1123–1134.
- Weatherburn, M.W.; Lubochinsky, B.; Zalta, J.P.; St, B. Phenol-Hypochlorite Reaction for Determination of Ammonia; UTC, 1954; Vol. 36;.
- Ito, Y. Golden Rules and Pitfalls in Selecting Optimum Conditions for High-Speed Counter-Current Chromatography. J Chromatogr A 2005, 1065, 145–168, doi:10.1016/j.chroma.2004.12.044.
- Hubert, J.; Plé, K.; Hamzaoui, M.; Renault, J.H. Polyphenol Purification by Solid Support-Free Liquid—Liquid Chromatography (CCC, CPC). In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer Berlin Heidelberg, 2013; pp. 2145–2172 ISBN 9783642221446.
- Xu, M.; Liu, P.; Jia, X.; Zhai, M.; Zhou, S.; Wu, B.; Guo, Z. Metabolic Profiling Revealed the Organ‐specific Distribution Differences of Tannins and Flavonols in Pecan. Food Sci Nutr 2020, 8, 4987–5006, doi:10.1002/fsn3.1797.
- Rainard, J.M.; Pandarakalam, G.C.; McElroy, S.P. Using Microscale Thermophoresis to Characterize Hits from High-Throughput Screening: A European Lead Factory Perspective. SLAS Discovery 2018, 23, 225–241.
- Nasreddine, R.; Nehmé, R. Microscale Thermophoresis for Studying Protein-Small Molecule Affinity: Application to Hyaluronidase. Microchemical Journal 2021, 170, 106763, doi:10.1016/j.microc.2021.106763.
- Hellinen, L.; Bahrpeyma, S.; Rimpelä, A.K.; Hagström, M.; Reinisalo, M.; Urtti, A. Microscale Thermophoresis as a Screening Tool to Predict Melanin Binding of Drugs. Pharmaceutics 2020, 12, 1–13, doi:10.3390/pharmaceutics12060554.
- Asmari, M.; Ratih, R.; Alhazmi, H.A.; El Deeb, S. Thermophoresis for Characterizing Biomolecular Interaction. Methods 2018, 146, 107–119, doi:10.1016/j.ymeth.2018.02.003.
- Wang, W. Protein Aggregation and Its Inhibition in Biopharmaceutics. Int J Pharm 2005, 289, 1–30, doi:10.1016/j.ijpharm.2004.11.014.

