RATIONAL DESIGN AND CONVENIENT SYNTHESIS OF TWO NOVEL FERROCENE-BASED SULFONYL DIAMINE PRECURSORS

- Cyclic voltammetry,
- ferrocene,
- organometallic,
- sulfonamide,
- X-ray
Copyright (c) 2023 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
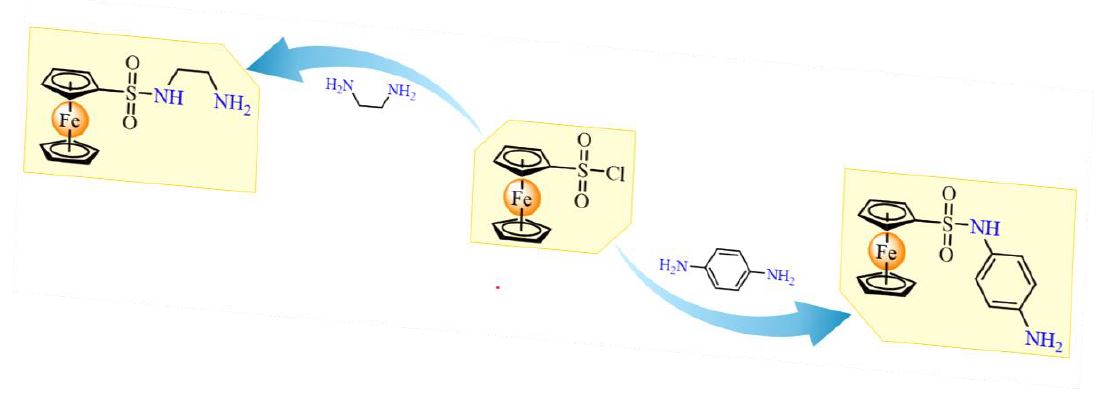
In the search of new organometallic precursors, this contribution describes a convenient synthesis to obtained new ferrocene-based sulfonyl diamine derivatives. The [(η5-C5H4SO2NH-CH2-CH2-NH2)Fe(η5-C5H5)] (1) and [(η5-C5H4SO2NH-C6H4-NH2)Fe(η5-C5H5)] (2) compounds were prepared by the reaction between (η5-C5H4SO2Cl)Fe(η5-C5H5) and the respective diamine precursor: ethylenediamine (1) or p-phenylenediamine (2) in good yields (86% for 1; 70% for 2). Both compounds were characterized by conventional spectroscopic techniques (infrared spectroscopy, nuclear magnetic resonance spectroscopy, mass spectrometry and elemental analysis) and cyclic voltammetry. In addition, the molecular structure of 1 was determined by single-crystal X-ray diffraction.
References
- H. Werner, Angew. Chem. Int. Ed., 51, 6052, (2012)
- R. Peters; D. F. Fischer; S. Jautze, in: Iron Catalysis: Fundamentals and Applications, B. Plietker Ed., Springer Berlin Heidelberg, Berlin, Heidelberg, 2011; 139.
- A. Khan; L. Wang; H. Yu; M. Haroon; R. S. Ullah; A. Nazir; T. Elshaarani; M. Usman; S. Fahad; F. Haq, Appl. Organomet. Chem., 32, e4575, (2018)
- P.-O. Schwartz; S. Förtsch; E. Mena-Osteritz; D. Weirather-Köstner; M. Wachtler; P. Bäuerle, RSC Adv., 8, 14193, (2018)
- M. Patra; G. Gasser, Nat. Rev. Chem., 1, 0066, (2017)
- J. S. Miller; A. J. Epstein, Angew. Chem. Int. Ed., 33, 385, (1994)
- P. Nguyen; P. Gómez-Elipe; I. Manners, Chem. Rev., 99, 1515, (1999)
- A. Sola; A. Tárraga; P. Molina, Dalton Trans., 41, 8401, (2012)
- Y. Wang; X. He; K. Wang; X. Ni; J. Su; Z. Chen, Biosens. Bioelectron., 32, 213, (2012)
- L.-Z. Du; J.-F. Gong; C. Xu; Y. Zhu; Y.-J. Wu; M.-P. Song, Inorg. Chem. Commun., 9, 410, (2006)
- F. Yang; X. Cui; Y.-n. Li; J. Zhang; G.-r. Ren; Y. Wu, Tetrahedron. 63, 1963, (2007)
- J. Zhang; L. Zhao; M. Song; T. C. W. Mak; Y. Wu, J. Organomet. Chem., 691, 1301, (2006)
- O. N. Chupakhin; I. A. Utepova; I. S. Kovalev; V. L. Rusinov; Z. A. Starikova, Eur. J. Org. Chem., 2007, 857, (2007)
- R. Pandey; R. K. Gupta; M. Shahid; B. Maiti; A. Misra; D. S. Pandey, Inorg. Chem., 51, 298, (2012)
- K. Yoshida; R. Yasue, Chem. Eur. J., 24, 18575, (2018)
- M. M. Abd-Elzaher; S. A. Moustafa; A. A. Labib; H. A. Mousa; M. M. Ali; A. E. Mahmoud, Appl. Organomet. Chem., 26, 230, (2012)
- J. Guillon; S. Moreau; E. Mouray; V. Sinou; I. Forfar; S. B. Fabre; V. Desplat; P. Millet; D. Parzy; C. Jarry; P. Grellier, Bioorg. Med. Chem., 16, 9133, (2008)
- C. Herrmann; P. F. Salas; B. O. Patrick; C. de Kock; P. J. Smith; M. J. Adam; C. Orvig, Dalton Trans., 41, 6431, (2012)
- K. Kowalski; A. Koceva-Chyła; A. Pieniążek; J. Bernasińska; J. Skiba; A. J. Rybarczyk-Pirek; Z. Jóźwiak, J. Organomet. Chem., 700, 58, (2012)
- L. Soulère; J. Bernard, Bioorg. Med. Chem. Lett., 19, 1173, (2009)
- K. N. Tiwari; J.-P. Monserrat; A. Hequet; C. Ganem-Elbaz; T. Cresteil; G. Jaouen; A. Vessières; E. A. Hillard; C. Jolivalt, Dalton Trans., 41, 6451, (2012)
- D. R. van Staveren; N. Metzler-Nolte, Chemical Reviews. 104, 5931, (2004)
- C.-W. Wei; Y. Peng; L. Zhang; Q. Huang; M. Cheng; Y.-N. Liu; J. Li, Bioorg. Med. Chem. Lett., 21, 5818, (2011)
- A. Mahajan; L. Kremer; S. Louw; Y. Guéradel; K. Chibale; C. Biot, Bioorg. Med. Chem. Lett., 21, 2866, (2011)
- J. Brichet; R. Arancibia; E. Berrino; C. T. Supuran, J. Enzyme Inhib. Med. Chem., 35, 622, (2020)
- D. Can; B. Spingler; P. Schmutz; F. Mendes; P. Raposinho; C. Fernandes; F. Carta; A. Innocenti; I. Santos; C. T. Supuran; R. Alberto, Angew. Chem. Int. Ed., 51, 3354, (2012)
- C. Concha; C. Quintana; A. H. Klahn; V. Artigas; M. Fuentealba; C. Biot; I. Halloum; L. Kremer; R. López; J. Romanos; Y. Huentupil; R. Arancibia, Polyhedron. 131, 40, (2017)
- Y. Huentupil; L. Peña; N. Novoa; E. Berrino; R. Arancibia; C. T. Supuran, J. Enzyme Inhib. Med. Chem., 34, 451, (2019)
- C. Quintana; G. Silva; A. H. Klahn; V. Artigas; M. Fuentealba; C. Biot; I. Halloum; L. Kremer; N. Novoa; R. Arancibia, Polyhedron. 134, 166, (2017)
- A. J. Salmon; M. L. Williams; Q. K. Wu; J. Morizzi; D. Gregg; S. A. Charman; D. Vullo; C. T. Supuran; S.-A. Poulsen, J. Med. Chem., 55, 5506, (2012)
- Z. H. Chohan, J. Enzyme Inhib. Med. Chem., 24, 169, (2009)
- K. Chanawanno; T. S. Blesener; B. R. Schrage; V. N. Nemykin; R. S. Herrick; C. J. Ziegler, J. Organomet. Chem., 870, 121, (2018)
- K. Chanawanno; C. Holstrom; L. A. Crandall; H. Dodge; V. N. Nemykin; R. S. Herrick; C. J. Ziegler, Dalton Trans., 45, 14320, (2016)
- K. Chanawanno; C. Holstrom; V. N. Nemykin; R. S. Herrick; C. J. Ziegler, ChemistrySelect. 1, 6438, (2016)
- Y. Huentupil; P. Chung; N. Novoa; R. Arancibia; P. Roussel; J. Oyarzo; A. H. Klahn; C. Silva; C. Calvis; R. Messeguer; R. Bosque; C. López, Dalton Trans., 49, 12249, (2020)
- C. Chen; T. M. J. Anselment; R. Fröhlich; B. Rieger; G. Kehr; G. Erker, Organometallics. 30, 5248, (2011)
- T. Homann-Müller; E. Rieger; A. Alkan; F. R. Wurm, Polym. Chem., 7, 5501, (2016)
- J. Leonard; B. Lygo; G. Procter. Advanced Practical Organic Chemistry, Third Edition, Taylor & Francis, 2013.
- L. Palatinus; G. Chapuis, J. Appl. Crystallogr., 40, 786, (2007)
- O. V. Dolomanov; L. J. Bourhis; R. J. Gildea; J. A. K. Howard; H. Puschmann, J. Appl. Crystallogr., 42, 339, (2009)
- I. Almendras; Y. Huentupil; N. Novoa; P. Roussel; D. R. Melis; G. S. Smith; R. Arancibia, Inorganica Chim. Acta. 496, 119050, (2019)
- J. L. Cavill; R. L. Elliott; G. Evans; I. L. Jones; J. A. Platts; A. M. Ruda; N. C. O. Tomkinson, Tetrahedron. 62, 410, (2006)
- S. Şenkardeş; M. İ. Han; N. Kulabaş; M. Abbak; Ö. Çevik; İ. Küçükgüzel; Ş. G. Küçükgüzel, Mol. Divers., 24, 673, (2020)
- I. Cârlescu; D. Scutaru; N. Hurduc; O. Cătănescu; L.-C. Chien, Mol. Cryst. Liq. Cryst 439, 107/[1973], (2005)
- J. Gómez; A. Hugo Klahn; M. Fuentealba; D. Sierra; C. Olea-Azar; M. E. Medina, Inorg. Chem. Commun., 61, 204, (2015)
- B. J. Barron; W.-T. Wong; P. Chiu; K. K. Hii, ACS Catal., 6, 4189, (2016)
- M. R. Jain; S. Shetty; G. Chakrabarti; V. Pandya; A. Sharma; B. Parmar; S. Srivastava; M. Raviya; H. Soni; P. R. Patel, Eur. J. Med. Chem., 43, 880, (2008)
- A. B. Gündüzalp; G. Parlakgümüş; D. Uzun; Ü. Ö. Özmen; N. Özbek; M. Sarı; T. Tunç, J. Mol. Struct., 1105, 332, (2016)
- N. Özbek; S. Alyar; H. Alyar; E. Şahin; N. Karacan, Spectrochim. Acta A Mol. Biomol. Spectrosc., 108, 123, (2013)
- M. Č. Semenčić; I. Kodrin; K. Molčanov; M. Kovačević; V. Rapić, Heliyon. 8, e09470, (2022)
- A. J. Bard; L. R. Faulkner; H. S. White. Electrochemical Methods: Fundamentals and Applications, Wiley, 2022.