THEORETICAL STUDY OF THE BONDING BETWEEN ALGINATE AND GRAPHENE OXIDE THROUGH THE INTERACTION OF ITS CARBOXYLIC GROUPS WITH THE DIVALENT METALS Cu2+, Co2+, Zn2+, AND Mn2+.
- Alginate-graphene oxide mixture,
- adsorption metals,
- DFT,
- NBO,
- ELF
- QTAIM ...More
Copyright (c) 2023 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
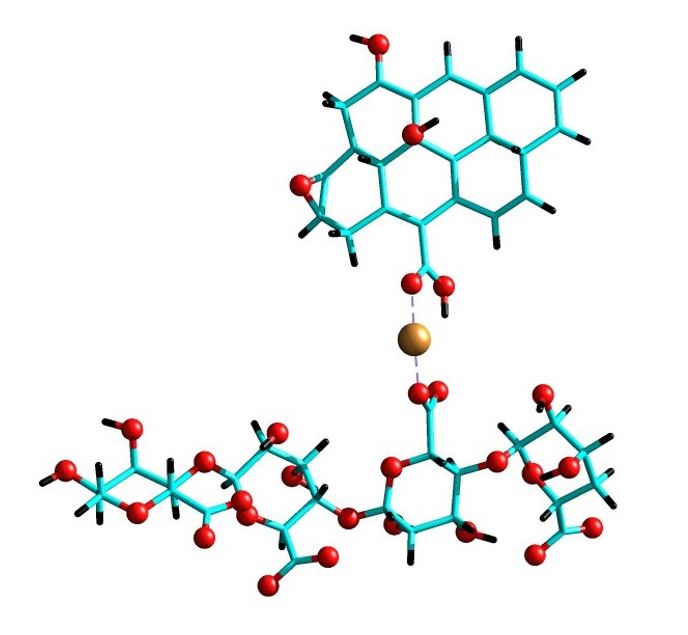
In the present study, the interaction of transition cations in their divalent states Cu2+, Co2+, Zn2+ and Mn2+, which act as bridges when interacting with carboxylic groups of alginate and graphene oxide, was studied. The chemical nature of the interactions under study was determined using the natural bond orbitals (NBO) method, topological analysis of the electronic location function (ELF), and the quantum theory of atoms in molecules (QTAIM). The results showed that the interactions between the metals under study with the carboxylic groups of alginate and graphene oxide are of the coordinated type with a high electrostatic component.
References
2. Rhimi A, Zlaoui K, Horchani-Naifer K, Ennigrou DJJIPJ. (2022). Characterization and extraction of sodium alginate from Tunisian algae: synthesizing a cross-linked ultrafiltration membrane. Iran. Polym. J.31:367-82
3. Shaikh MAJ, Alharbi KS, Almalki WH, Imam SS, Albratty M, et al. (2022). Sodium alginate based drug delivery in management of breast cancer. Carbohydr. Polym.119689
4. Gao X, Guo C, Hao J, Zhao Z, Long H, Li MJIjobm. (2020). Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 164:4423-34.
5. Sanchez-Ballester NM, Bataille B, Soulairol IJCP. (2021). Sodium alginate and alginic acid as pharmaceutical excipients for tablet formulation: Structure-function relationship.
Carbohydr. Polym. 270:118399.
6. Martinsen A, Skjåk‐Bræk G, Smidsrød OJB, bioengineering. (1989). Alginate as immobilization material: I. Correlation between chemical and physical properties of alginate gel beads. Biotechnol. Bioeng.33:79-89.
7. Johnson FA, Craig DQ, Mercer ADJJop, pharmacology. (1997). Characterization of the block structure and molecular weight of sodium alginates. J. Pharm. Pharmacol. 49:639-43.
8. Fu S, Thacker A, Sperger DM, Boni RL, Buckner IS, et al. (2011). Relevance of rheological properties of sodium alginate in solution to calcium alginate gel properties. AAPS PharmSciTech. 12:453-60
9. Ngah WW, Fatinathan SJCEJ. (2008). Adsorption of Cu (II) ions in aqueous solution using chitosan beads, chitosan–GLA beads and chitosan–alginate beads. Chem. Eng. J. 143:62-72
10. Gokila S, Gomathi T, Sudha P, Anil SJIjobm. (2017). Removal of the heavy metal ion chromiuim (VI) using Chitosan and Alginate nanocomposites. Int. J. Biol. Macromol. 104:1459-68.
11. Yadav M, Rhee KY, Park SJCp. (2014). Synthesis and characterization of graphene oxide/carboxymethylcellulose/alginate composite blend films. Carbohydr. Polym.110:18-25
12. Serrano-Aroca Á, Ruiz-Pividal J-F, Llorens-Gámez MJSr. (2017). Enhancement of water diffusion and compression performance of crosslinked alginate films with a minuscule amount of graphene oxide. Sci. Rep.7:1-8
13. Ionita M, Pandele MA, Iovu HJCp. (2013). Sodium alginate/graphene oxide composite films with enhanced thermal and mechanical properties. Carbohydr. Polym. 94:339-44
14. Suk JW, Piner RD, An J, Ruoff RSJAn. (2010). Mechanical properties of monolayer graphene oxide. ACS Nano. 4:6557-64
15. Zhu Y, Murali S, Cai W, Li X, Suk JW, et al. (2010). Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater.22:3906-24
16. Brisebois P, Siaj MJJoMCC. (2020). Harvesting graphene oxide–years 1859 to 2019: a review of its structure, synthesis, properties and exfoliation. J. Mater. Chem. C 8:1517-47
17. Li J, Ma J, Chen S, Huang Y, He JJMS, C E. (2018). Adsorption of lysozyme by alginate/graphene oxide composite beads with enhanced stability and mechanical property.
Mater. Sci. Eng. 89:25-32
18. Ji C, Yang S, Tao E, Cheng Y, Hao X, Li YJJoECE. (2021). Three-dimensional network graphene oxide/sodium alginate aerogel beads with slit-shaped structure: Synthesis, performance and selective adsorption mechanism for Cu (II). J. Environ. Chem. Eng.9:106819
19. Serrano-Aroca Á, Iskandar L, Deb SJEPJ. (2018). Green synthetic routes to alginate-graphene oxide composite hydrogels with enhanced physical properties for bioengineering applications. Eur. Polym. J.103:198-206
20. Sabater i Serra R, Molina-Mateo J, Torregrosa-Cabanilles C, Andrio-Balado A, Meseguer Dueñas JM, Serrano-Aroca ÁJP. (2020). Bio-nanocomposite hydrogel based on zinc alginate/graphene oxide: Morphology, structural conformation, thermal behavior/degradation, and dielectric properties. Polym.12:702
21. Algothmi WM, Bandaru NM, Yu Y, Shapter JG, Ellis AVJJoc, science i. (2013). Alginate–graphene oxide hybrid gel beads: An efficient copper adsorbent material. J. Colloid Interface Sci.397:32-8
22. Weinhold F, Landis C, Glendening EJIRiPC. (2016). What is NBO analysis and how is it useful? Int. Rev. Phys. Chem.35:399-440
23. Lin X, Wu W, Mo YJCCR. (2020). A theoretical perspective of the agostic effect in early transition metal compounds. Coord. Chem. Rev. 419:213401
24. Platts JA, Baker RJJDT. (2020). A computational investigation of orbital overlap versus energy degeneracy covalency in [UE 2] 2+(E= O, S, Se, Te) complexes. Dalton Trans. 49:1077-88
25. Liu S, Rong C, Lu T, Hu HJTJoPCA. (2018). Identifying strong covalent interactions with Pauli energy. J. Phys. Chem.122:3087-95
26. Fuentealba P, Chamorro E, Santos JC. (2007). Understanding and using the electron localization function. Theor. Comput. Chem.19:57-85
27. Savin A, Becke A, Flad J, Nesper R, Preuss H, Von Schnering HJACIEiE. (1991). A new look at electron localization. Angew. Chem. 30:409-12
28. Savin A, Nesper R, Wengert S, Fässler TFJACIEiE. (1997). ELF: The electron localization function. Angew. Chem. 36:1808-32
29. Chauvin R, Lepetit C, Silvi B, Alikhani E. (2016). Applications of topological methods in molecular chemistry. Springer
30. Rodríguez JIJJocc. (2013). An efficient method for computing the QTAIM topology of a scalar field: The electron density case. J. Comput. Chem. 34:681-6
31. Popelier PLA, Aicken F, O’Brien S. (2000). Atoms in molecules. Prentice Hall Manchester
32. Malcolm NO, Popelier PLJFd. (2003). The full topology of the Laplacian of the electron density: scrutinising a physical basis for the VSEPR model. Faraday Discuss.124:353-63
33. Popelier PJCCR. (2000). On the full topology of the Laplacian of the electron density. Coord. Chem. Rev. 197:169-89
34. Wang J-J, Chen X, Zhang B-S, Li C-B, Wang Y-FJJoMS. (2021). Experimental (X-ray, TGA) and computation (NBO, AIM) studies of Iron (II) complex with thiabendazole and 5-aminoisophthalate. J. Mol. Struct.1245:131100
35. Lu X, Duchimaza-Heredia J, Cui QJTJoPCA. (2019). Analysis of Density Functional Tight Binding with Natural Bonding Orbitals. J. Phys. Chem 123:7439-53
36. Lee LP, Cole DJ, Payne MC, Skylaris CKJJocc. (2013). Natural bond orbital analysis in the ONETEP code: applications to large protein systems. J. Comput. Chem.34:429-44
37. Vektarienė AJLJoP. (2018). The transition metal to ligand bonding nature: a quantum chemical study of π-allyl-ruthenacycle molecule. Lith. J. Phys.58
38. Karafiloglou PJJoCC. (2001). A method to calculate the weights of nbo electronic structures from Moffitt's theorem. J. Comput. Chem. 22:306-15
39. Zülfikaroğlu A, Batı H, Dege NJJoMS. (2018). A theoretical and experimental study on isonitrosoacetophenone nicotinoyl hydrazone: Crystal structure, spectroscopic properties, NBO, NPA and NLMO analyses and the investigation of interaction with some transition metals.
J. Mol. Struct.1162:125-39
40. Tsirelson V, Stash AJCpl. (2002). Determination of the electron localization function from electron density. Chem. Phys. Lett. 351:142-8
41. Nalewajski RF, Köster AM, Escalante SJTJoPCA. (2005). Electron localization function as information measure. J. Phys. Chem. A 109:10038-43
42. Kohout M, Wagner FR, Grin YJTCA. (2002). Electron localization function for transition-metal
compounds. Theor Chem Acc 108:150-6
43. Zou G, Jo H, Lim SJ, You TS, Ok KMJACIE. (2018). Rb3VO (O2) 2CO3: a four‐in‐one carbonatoperoxovanadate exhibiting an extremely strong second‐harmonic generation response.
Angew. Chem. 57:8619-22
44. Jerabek P, Schuetrumpf B, Schwerdtfeger P, Nazarewicz WJPRL. (2018). Electron and nucleon localization functions of oganesson: approaching the thomas-fermi limit. Phys. Rev. Lett. 120:053001
45. Mierzwa G, Gordon AJ, Berski SJJoMS. (2020). The nature of the triple BB, double, BB, single,
B–B, and one-electron, BB boron-boron bonds from the topological analysis of electron localisation
function (ELF) perspective. J. Mol. Struct.1221:128530.
46. Fuster F, Dézarnaud-Dandine C, Chevreau H, Sevin AJPCCP. (2004). A theoretical study of the
bonding in NO,(NO) 2,(NO) 2− and (NO) 2 2−using a topological analysis of the electron localization function. Phys. Chem. Chem. Phys. 6:3228-34.
47. Pilme J, Silvi B, Alikhani MEJTJoPCA. (2003). Structure and stability of M− CO, M= first transition-row metal: An application of density functional theory and topological approaches. J. Phys.Chem. A 107:4506-14.
48. Durlak P, Latajka Z, Berski SJTJocp. (2009). A Car–Parrinello and path integral molecular dynamics study of the intramolecular lithium bond in the lithium 2-pyridyl-N-oxide acetate. J. Phys.Chem. 131:024308
49. Pendás AM, Francisco E, Blanco MJCPL. (2008). Electron–electron interactions between ELF basins. Chem. Phys. Lett. 454:396-403
50. Savin A, Silvi B, Colonna FJCjoc. (1996). Topological analysis of the electron localization function applied to delocalized bonds. Can. J. Chem. 74:1088-96
51. Mierzwa G, Gordon AJ, Berski SJNJoC. (2018). On the nature of the boron–copper interaction.
Topological study of the electron localisation function (ELF). New J. Chem. 42:17096-114
52. Koumpouras K, Larsson JAJJoPCM. (2020). Distinguishing between chemical bonding and physical binding using electron localization function (ELF). J. Phys.: Condens. Matter 32:315502
53. Lepetit C, Kahn MLJRoCI. (2021). QTAIM and ELF topological analyses of zinc-amido complexes. Res. Chem. Intermed. 47:377-95
54. Mierzwicki K, Berski S, Latajka ZJCPL. (2000). Nature of chemical bonds in MCCH (M= Li, Na, K) based on the topological analysis of electron localisation function (ELF) and electron density. Chem. Phys. Lett. 331:538-46
55. Gassoumi B, Mehri A, Hammami H, Castro M, Karayel A, et al. (2022). Spectroscopic characterization, host-guest charge transfer, Hirshfeld surfaces, AIM-RDG and ELF study of adsorption and chemical sensing of heavy metals with new derivative of Calix [4] quinone: A DFT-D3 computation. Mater. Chem. Phys. 278:125555
56. Michalski M, Gordon AJ, Berski SJP. (2021). Theoretical insights and quantitative prediction of the nature of boron–chalcogen (O, S, Se, Te) interactions using the electron density and the electron localisation function (ELF). Polyhedron. 210:115495
57. Poater J, Duran M, Sola M, Silvi BJCr. (2005). Theoretical evaluation of electron delocalization in aromatic molecules by means of atoms in molecules (AIM) and electron localization function (ELF) topological approaches. Chem. Rev. 105:3911-47
58. Lu T, Chen FJTJoPCA. (2013). Bond order analysis based on the Laplacian of electron density in fuzzy overlap space. J. Phys. Chem. A. 117:3100-8
59. Korabel'nikov DV, Zhuravlev YNJRa. (2019). The nature of the chemical bond in oxyanionic crystals based on QTAIM topological analysis of electron densities. RSC Adv. 9:12020-33
60. Cukrowski I, Govender KK, Mitoraj MP, Srebro MJTJoPCA. (2011). QTAIM and ETS-NOCV analyses of intramolecular CH••• HC interactions in metal complexes. J. Phys. Chem. A 115:12746-57
61. Kazachenko AS, Akman F, Abdelmoulahi H, Issaoui N, Malyar YN, et al. (2021). Intermolecular hydrogen bonds interactions in water clusters of ammonium sulfamate: FTIR, X-ray diffraction, AIM, DFT, RDG, ELF, NBO analysis. J. Mol. Liq. 342:117475
62. Louit G, Hocquet A, Ghomi M, Meyer M, Sühnel JJP. (2003). Guanine tetrads interacting with metal ions. An AIM topological analysis of the electronic density. PhysChemComm. 6:1-5
63. Arnold WD, Oldfield EJJotACS. (2000). The chemical nature of hydrogen bonding in proteins via NMR: J-couplings, chemical shifts, and AIM theory. J. Am. Chem. Soc. 122:12835-41
64. Cukrowski I, de Lange JH, Mitoraj MJTJoPCA. (2014). Physical nature of interactions in ZnII complexes with 2, 2′-bipyridyl: Quantum theory of atoms in molecules (QTAIM), interacting quantum atoms (IQA), noncovalent interactions (NCI), and extended transition state coupled with natural orbitals for chemical valence (ETS-NOCV) comparative studies. J. Phys. Chem. A 118:623-37
65. Li L, Wu C, Wang Z, Zhao L, Li Z, et al. (2015). Density functional theory (DFT) and natural bond orbital (NBO) study of vibrational spectra and intramolecular hydrogen bond interaction of l-ornithine–l-aspartate. Spectrochim. Acta, Part A. 136:338-46
66. Glendening ED, Landis CR, Weinhold FJWircms. (2012). Natural bond orbital methods. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2:1-42
67. Zhou Y, Pan S, Dong X, Wang L, Zhou M, Frenking GJJotACS. (2022). Generation and Characterization of the Charge-Transferred Diradical Complex CaCO2 with an Open-Shell Singlet Ground State. J. Am. Chem. Soc. 144, 18, 8355–8361
68. Pan L, Wang Z, Yang Q, Huang RJN. (2018). Efficient removal of lead, copper and cadmium ions from water by a porous calcium alginate/graphene oxide composite aerogel.
Nanomaterials. 8:957
69. Abd-Elhamid A, Elgoud E, Aly HJC. (2022). Alginate modified graphene oxide for rapid and effective sorption of some heavy metal ions from an aqueous solution. Cellulose. 1-15
70. Yang X, Zhou T, Ren B, Hursthouse A, Zhang YJSr. (2018). Removal of Mn (II) by sodium alginate/graphene oxide composite double-network hydrogel beads from aqueous solutions.
Sci. Rep. 8:1-16
71. Bai C, Wang L, Zhu ZJIjobm. (2020). Adsorption of Cr (III) and Pb (II) by graphene oxide/alginate hydrogel membrane: Characterization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 147:898-910
72. Priya VN, Rajkumar M, Mobika J, Sibi SLJPEL-dS, Nanostructures. (2021). Alginate coated layered double hydroxide/reduced graphene oxide nanocomposites for removal of toxic As (V) from wastewater. Phys. E (Amsterdam, Neth.). 127:114527
73. Sun Y, Li Y, Chen B, Cui M, Xu W, et al. (2022). High‐Efficiency Adsorption Performance of Cobalt Alginate/Graphene Oxide Aerogel Prepared by Green Method for Methylene Blue. ChemistrySelect. 7:e202201216
74. Zheng H, Yang J, Han SJJoAPS. (2016). The synthesis and characteristics of sodium alginate/graphene oxide composite films crosslinked with multivalent cations. J. Appl. Polym. Sci.133