EFFECT OF PROCESSING AND PRESERVATION METHODS ON TOTAL PHENOLIC CONTENTS AND ANTIOXIDANT ACTIVITIES OF GARLIC (ALLIUM SATIVUM)

- DPPH,
- Garlic,
- Phenolics,
- Flavonoids,
- Preservation methods
- Antioxidant activity ...More
Copyright (c) 2023 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
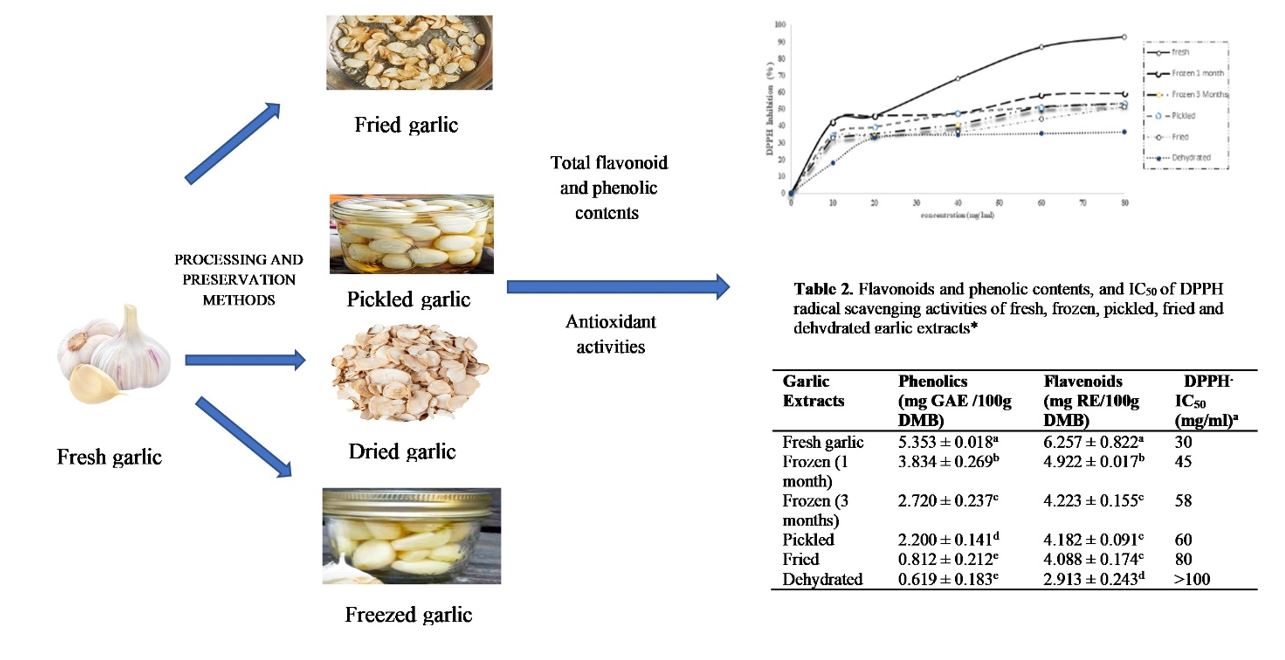
Garlic (Allium sativum L.) is one of the most commonly produced vegetables worldwide. It has been consumed as a species and is considered important medicine for treating and preventing many diseases due to its content of various bioactive phytomolecules. This study aimed to assess the effect of different processing and preservation methods, including pickling, frying, freezing, and drying, on total phenolics and antioxidant activities of ethanolic extracts of garlic. Total phenolics, flavonoids, and antioxidant activities of dehydrated, pickled, and fried garlic were measured directly after treatment, and frozen garlic was assessed after 1 and 3 months of storage. Results showed that the total flavonoids and phenolics contents, and antioxidant activities of all treated samples significantly decreased compared with fresh sample. Phenolic contents strongly correlated with DPPH radical scavenging activities (r=0.956) at a concentration of 40 mg/mL for all treatments. The reducing power activity result of fresh garlic sample at a concentration of 20 mg/mL was 213.9% (30µg vitamin c equivalent); other treatments were shown to have significantly lower reducing power activities than fresh samples. Flavonoid contents showed a strong correlation with reducing power activities (r=0.759) at a concentration of 40 mg/mL for all treatments, while in phenolic contents, the correlation (r) was 0.94 at the same concentration. It can be concluded that fresh garlic showed the highest reducing power activities compared to the other treatments, while lowest activity was for dehydrated garlic. In addition, freezing process resulted in the highest preservation of the total flavonoid and phenolic contents and antioxidant activities.
References
- References
- T. Satn, G. Miyata, Pharma Nutr. 16, 787-788, (2000)
- C. Yiannakopoulou, J. Cardiovasc. Pharmacol. Ther. 17(4), 366–372, (2012)
- N, Morihara, M. Hayama, H. Fujii, Plant Foods Hum. Nutr. 66(1), 17-21, (2011)
- N. Morihara, N. Ide, N. Weiss, Phytother Res. 24(4), 602–608, (2010)
- H. Çubukçu, N. Kılıçaslan, I. Durak, Sao Paulo Med. J. 137 (05), 407-413, (2019)
- Y. Queiroz, Y. Ishimoto, M. Bastos, R. Sampaio, S. Torres, Food Chem. 115(1), 371-374, (2009)
- N. Ide, H. Lau, J. Pharm. Pharmaco. 49(9), 908–911, (1997)
- H. Park, K. Park, E. Park, Plant Foods for Hum. Nutr. 64(4), 244-249, (2009)
- N. Weiss, L. Papatheodorou, N. Morihara, R. Hilge, N. Ide, J. Ethnopharmaco. 145(1), 162-167, (2013)
- M. Blumenthal, A. Goldberg, J. Brinkman, American Botanical Council, 130-133, (2000)
- K. Song, J.A. Milner, J. Nutr. 131, 10545-10575, (2001)
- M. Corzo, N. Corzo, M. Villamiel, Trends Food Sci. Technol. 18(12), 609-625, (2007)
- V. Lanzotti, J. Chromatogra. 1112(1), 3-22, (2006)
- V, Beato, F., Orgaz, F. Mansilla, A. Montano, Plant Foods Hum. Nutr. 66(3), 218-223, (2011)
- D. Waterer, D. Schmitz, Can. J. Plant Sci. 74(3), 611–614, (1994)
- M. Zakia, A. Nihal, Bio. Chem. Sci. 5(4), pp.588, (2013)
- H. Delaviz, A. Mirazaei, H. Mohammadi, World J Pharm Pharm Sci. 3(7), 242-252, (2014)
- A. Patras, N. Brunton, B. Tiwari, F. Butler, Food Chem., 114, 484-491, (2009)
- J. Song, G. An, C. Kim, Food chem. 83, 69-74, (2003)
- Z. Qiu, Z. Zheng, B. Zhang, D. Sun-Waterhouse, Compr Rev Food Sci Food Saf. 1–34, (2020)
- J. Hur, Y. Lee, C. Kim, I. Choi, B. Kim, Food Chem. 160, 346-356, (2014)
- E. Chan, Y. Lim, S. Wong, K. Lim, S. Tan, M. Yong, Food Chem. 113, 166-172, (2009)
- H. Volden, A. Borge, M. Hansen, T. Wicklund, B. Bengtsson, LWT-Food Sci Technol. 42, 63-73, (2006)
- W. Zheng, X. Shi, S. Da-Wen, Drying Technol. 21(7), 1173-1184, (2003)
- L. Said, H. Najja, M. Neffati, S. Bellagha, J Food Quality, 36(6), 403-410, (2013)
- Y. Lim, J. Murtijaya, LWT-Food Sci Technol. 40, 1664-1669, (2007)
- P. Duh, G. Yen, Food Chem. 60, 639-645, (1997)
- G., Miliuskas, P. Venskutnis, T. Van, Food Chem. 85, 231-237, (2004)
- T. Hatano, H. Kagawa, T. Yasuhara, T. Okuda, Chem. Physic. Bull. 36, 2009-2097, (1988)
- A. Yildirm, A. Mavi, K. Aydan, J Sci Food Agr. 83, 64-69, (2003)
- E. Odebunmi, O. Oluwaniyi, M. Bashiru, J Appl. Sci Res. 6(3), 272-274, (2010)
- P. Patricia, C. Henrique, A. Souza, V. Silva, M. Pacheco, Food Sci Technol. 34(3), 4-8, (2014)
- D. Lawson, Z. Wang, B. Huges, Plant med. 57(4), 363-370, (1991)
- B. Hsu, F. Anwar, R. Przybylski, Food Chem. 1046, 1106-1114, (2007)
- B. Sultana, F. Anwar, S. Iqbal, Int. J. Food Sci. Technol., 43, 560-567, (2008)
- H. Wu, T. Haig, J. Prately, D. Lemerle, M. An, J Chromatogr. 864, 315-321, (1999)
- B. Bonzin, N. Mimica-Dukic, N. Samojlik, I. Goran, R. Igic, Food Chem, 111(4), 925-929, (2008)
- S. Lee, A. Coates, Food Chem. 65, 165-168, (1999)
- G. Wang, Doctoral Dissertation, Ohio University, (2013)
- B. De Ancos, E.M. González, M.P. Cano, J Agric. Food Chem. 48 (10), 4565-4570, (2000)
- N. Emy, B. Corinne, A. Sandra, R. Katia, J Food Nutr. Sci. 6, 299-313, (2015)
- P. Lima, C. Lopes, M. Rossetto, F. Vianello, Int. J Food Sci. Technol. 44, 1118-1124, (2009)
- N. Turkmen, F. Sari, Y. Velioglu, Food Chem. 93, 713-718, (2009)
- W. Somsub, R. Kongkachuichai, P. Sungpuag, R. Charoensiri, J Food Comp. Anal. 21, 187-197, (2008)
- A. Korus, LWT-Food Sci Technol. 44, 1711-1716, (2011)
- C. Nicoli, M. Anese, M. Parpinel, Trends Food Sci. Technol., 10, 94-100, (1999)
- F.S. Kübra, S.A. Burçin, J Food Health Sci. 1(3): 135-141, (2015)
- M. Atanassova, S. Georgieva, K. Ivancheva, J Uni. Chem. Technol. Metallurgy, 46(1), 81-88, (2011)
- G. Petzold, M. Aguilera, Food Biophys. 4(4), 378-396, (2009)
- N. Harbourne, E. Marete, J. Jacquier, D. Oriordan, LWT-Food Sci. Technol. 42, 1468-1473, (2009)
- T. Rababah, M. Alhamad, M. AL-Mahasneh, K. Ereifej, J. Andrade, W. Yang, Int. J. Agric. Biol. Eng. 8(2), 145-150, (2015)
- A. Schieber, G. Williamson, J Nutr. 130(8), 2073-2085, (2001)
- N. Prabha, V. Patwardhan, J. Biosci. 4(1), 69-78, (1982)
- S. Yara, D. Queiroz, B., Patricia, S.J, Antunes, G.R. Vicente, J.S., Sampaio, H. Deborah, M. E.F. Bastos, S. Torres, Int. J. Food Sci. Technol. 49(5), 1308-1314, (2013)
- K. Prasad, A. Laxdal, M. Yu, L. Raney, Mol. Cell. Biochem. 154, 55-63, (1996)
- B. Cho, S. Xu, Compara. Biochem. Phys. 126, 195–201, (2000)
- T. Mazzeo, D. N’Dri, E. Chiavaro, A. Visconti, V. Fogliano, N. Pellegrini, Food Chem. 50(12), 3444-3452, (2011)
- N. Cecilia, Stewart Postharvest Rev. 4(4), 1-14, (2008)
- D. Gupta, R. Bhardwaj, K. Gupta, Afr. J. Tradit. Complement. Altern. Med. 8(4), 391-397, (2011)
- S. Arabshahi, D. Vishalakshi, A. Urooja, Food Chem. 10, 1100-1105, (2007)
- S. Karakaya, Crit. Rev. Food Sci. Nutr. 44, 453-464, (2004)
- D. Zhang, Y. Hamauazu, Food Chem. 88, 503-509, (2004)
- C. Gertz, S. Klostermann, P. Kochhar, Eur J Lipid Sci Technol, 102, 543-551, (2000)
- S. Velioglu, G. Mazza, L. Gao, B. Oomah, J Agric. Food Chem. 46, 4113-4117, (1998)
- E. Gonzalez, B. de Ancos, M. Cano, J. ScI. Food Agric. 83(7), 722-726, (2003)
- W. Zheng, S. Wang, J Agric. Food Chem. 49, 5165-5170, (2001)