DETERMINATION OF IBUPROFEN AND 1-HYDROXYIBUPROFEN IN AQUEOUS SAMPLES USING CORK AS A NATURAL PHASE IN ROTATING-DISK SORPTIVE EXTRACTION

- Cork, ibuprofen, 1-hydroxyibuprofen, natural sorptive phase, RDSE.
Copyright (c) 2023 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
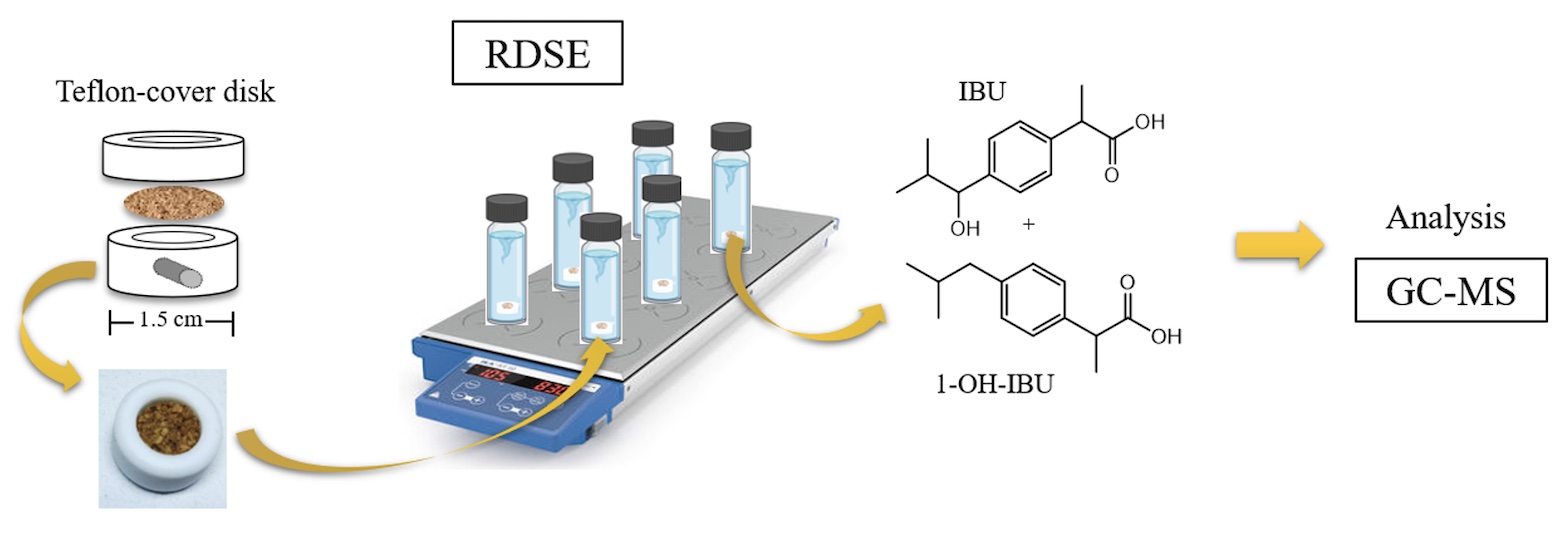
Ibuprofen is one of the most widely used nonsteroidal anti-inflammatory drugs due to its analgesic, anti-inflammatory and antipyretic properties, as well as its low cost and easy accessibility. A fraction of the compound and its metabolites are excreted in the urine, being eliminated in the wastewater reaching river waters in the range of ng L-1 to µg L-1. In this context, highly sensitive and selective analytical methods are required to quantify them, including these methods a pre-concentration step. In this work, the use of a microextraction technology based on rotating-disk sorptive extraction, involving a sorptive phase of laminar cork, was implemented for the extraction of ibuprofen and 1-hydroxyibuprofen from aqueous samples and their subsequent determination by gas chromatography coupled to mass spectrometry.
The optimal conditions for determination of the analytes were: 20 mL of sample volume, pH 2, 20 % w/v NaCl (to increase the ionic strength), 90 min of extraction time and 2000 rpm of rotation velocity of the disk. Recoveries of 118 and 39 % and relative standard deviations of 6 and 13 % for ibuprofen and 1-hydroxyibuprofen were obtained, respectively. The presence of both compounds in river waters (Mapocho River, Santiago de Chile) at a concentration of 2.56 to 4.08 µg L-1 were found. The use of laminar cork as a natural sorbent phase immobilized in the rotating-disk allowed to extract the analytes from water samples through its lipophilic-hydrophilic balance that favors the interaction with the compounds under study.
References
- Q. Bu, B. Wang, J. Huang, S. Deng, G. Yu, J. Hazard. Mater. 262, 189-211, (2013).
- K. Kümmerer, Annu. Rev. Environ. Resour. 35, 57-75, (2010).
- L. H. Santos, A. N. Araújo, A. Fachini, A. Pena, C. Delerue-Matos, M. Montenegro, J. Hazard. Mater. 175, 45-95, (2010).
- N. A. Khan, S. U. Khan, S. Ahmed, I. H. Farooqi, M. Yousefi, A. A. Mohammad, F. Changani, TrAC-Trend Anal. Chem. 122, 115744, (2020).
- K. Kümmerer, J. Environ. Manage. 90, 2354-2366, (2009).
- F. Méndez-Arriaga, S. Esplugas, J. Giménez, Water Res. 44, 589-594, (2010).
- Instituto de Salud Pública de Chile, Gob.cl. (2021). Available at: https://www.ispch.cl/noticia/isp-informa-sobre-los-medicamentos-mas-vendidos-durante-el-ano-2021/. Accessed August 28th, 2022.
- R. Bushra, N. Aslam, Oman Med. J. 25, 155-161, (2010).
- H. R. Buser, T. Poiger, M. D. Müller, Environ. Sci. Technol. 33, 2529-2535, (1999).
- I. Neunzig, A. Göhring, C. A. Drăgan, J. Zapp, F. T. Peters, H. H. Maurer, M. Bureik, J. Biotechnol. 157, 417-420, (2012).
- K. B. Borges, A. R. Moraes de Oliveira, T. Barth, V. A. Polizel, M. T. Pupo, P. S. Bonato, Anal. Bioanal. Chem. 399, 915-925, (2011).
- L. Ferrando-Climent, N. Collado, G. Buttiglieri, M. Gros, I. Rodriguez-Roda, S. Rodriguez-Mozaz, D. Barceló, Sci. Total Environ. 438, 404-413, (2012).
- P. Richter, D. Arismendi, M. Becerra-Herrera, TrAC-Trend Anal. Chem. 137, 116209, (2021).
- V. Manzo, J. Goya-Pacheco, D. Arismendi, M. Becerra-Herrera, A. Castillo-Aguirre, R. Castillo-Felices, M. Rosero-Moreano, E. Carasek, P. Richter, Anal. Chim. Acta, 1087, 1-10, (2019).
- C. M. S. Vieira, M. Mazurkievicz, A. M. Lopez, V. Debatin, G. A. Micke, P. Richter, M. Rosero-Moreano, E. Carasek, J. Sep. Sci. 41, 4047-4054, (2018).
- C. M. S. Vieira, G. Mafra, R. Brognoli, P. Richter, M. Rosero-Moreano, E. Carasek, Talanta, 208, 120459, (2020).
- A. M. A Pintor, C. I. A. Ferreira, J. C. Pereira, P. Correia, S. P. Silva, V. J. P. Vilar, C. M. S. Botelho, R. A. R. Boaventura. Water Res. 46, 3152-3166, (2012).
- S. P. Silva, M. A. Sabino, E. M. Fernandes, V. M. Correlo, L. F. Boesel, R. L. Reis, Int. Mater. Rev. 50, 345-365, (2005).
- I. Cerrato, A. Molina-Balmaceda, D. Arismendi, I. Ahumada, P. Richter, Green Anal. Chem. 1, 100008, (2022).
- A. Burcea, I. Boeraş, C. M. Mihut, D. Bănăduc, C. Matei, A. Curtean- Bănăduc, Sustainability, 12, 10197, (2020).
- A. N. Días, V. Simão, J. Merib, E. Carasek, Anal. Chim. Acta. 772, 33-39, (2013).
- Y. Corrotea, K. Sánchez, M. A. Rubio, P. Richter, J. Chil. Chem. Soc. 59, 2477-2480, (2014).
- V. Manzo, L. Honda, O. Navarro, L. Ascar, P. Richter, Talanta, 128, 486-492, (2014).
- M. Becerra-Herrera, L. Honda, P. Richter, J. Chromatogr. A.1423, 96-103, (2015).
- P. Richter, C. Leiva, C. Choque, A. Giordano, B. Sepúlveda, J. Chromatogr. A. 1216, 8598-8602, (2009).
- M. G. Arriagada, D. Pino, P. Richter, I. Toral, J. Chil. Chem. Soc. 66, 5035-5040, (2021).
- U. M. F. M Cerqueira, M. A. Bezerra, S. L. C. Ferreira, R. de Jesus Araújo, B. N. da Silva, C. G. Novaes, Food Chem. 364, 130429, (2021).

