DYNAMIC OF INDOLE ALKALOIDS IN A SOIL AND ITS RELATIONSHIPS WITH ALLELOPATHIC PROPERTIES

- Barley, Allelopathy, Indole alkaloids, Gramine, Soil, HPLC, Sorption, Persistence.
Copyright (c) 2023 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Allelopathy is one of the alternatives used for integrated weed management (IWM), in order to minimize the use of synthetic herbicides. Allelopathy is defined as the effect produced by a chemical released by a donor plant on the development of another competitive recipient plant.
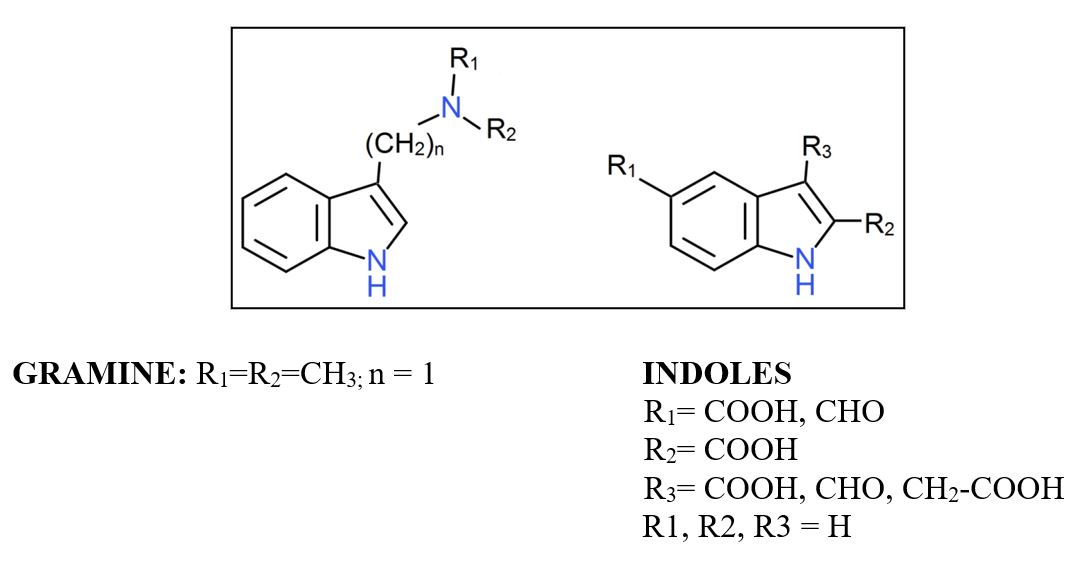
Research on allelopathic interactions has been focused on agricultural crops, and allelopathic activity of indole alkaloids has been reported in cereals such as barley (Hordeum vulgare L.), whose principal natural secondary metabolite is Gramine. The degradation products of this metabolite in agricultural soils have not been investigated, however indole and derivatives substituted in aromatic ring and heterocyclic can be produced, so the role of soil in the allelopathic behavior of these compounds is not yet clear.
In this work, phytotoxicity and dynamics of Gramine, indole and model series of substituted indoles in position 2 and 3 of the aromatic ring and position 5 of the aromatic ring were investigated in order to understanding the role of this metabolite in the allelopathy property of cereal barley.
The phytotoxic activity against competitive cereals and weeds was determined in soil, from which the percent inhibition (% I) of seed germination and seedling growth was measured. In adsorptions study, according to the values obtained from the adsorption coefficient (Kd), it was obtained that all the series of indole alkaloids shows a moderate adsorption in Alhue soil, with the exception of indole 3-acetic acid. In the study of desorption, this compound showed a desorption percentage of 81%, according to the Kd values obtained. The persistence studies indicated a half-life (t1/2) with a range of values between 7 h and 18 days for the series of indole alkaloids studied, where the highest value of t1/2 was for indole 2-carboxylic acid and the minor one for indole 5-carbaldehyde.
The dynamics of the compounds in Alhue soil affect phytotoxic activity, as well as bioavailability, so that soil plays an essential role in the phytotoxic effect of the compounds. Indole and Indole 2-carboxylic acid had the greatest phytotoxic effect, this behavior can be attributed to its greater persistence and low adsorption, that is, they are more bioavailable in Alhue soil and so the role as an allelochemical would be favorable.
References
- A.P. Appleby, F. Muller, S. Carpy, Weed control. In: Muller, F. (Ed.), Agrochemicals. Wiley, New York, pp. 687–707 (2000).
- A. Scavo, A. Restuccia, G. Mauromicale, Allelopathy: general principles and basic aspects for agroecosystem control. In: In: Gaba, S., Smith, B., Lichtfouse, E. (Eds.), m. Sustainable Agriculture Reviews, vol. 28. Springer, Cham, pp. 47–101 (2018a).
- E. L. Rice, 1984. Allelopathy, 2nd ed. Academic Press, Orlando (1984).
- F. A. Macias, J. M. Molinillo, R. M. Varela, J. C. Galindo, Pest Manag. Sci. 63: 327, (2007).
- J. E. Pratley, Plant Prot. Q. 11, 213, (1996).
- M. Ahn, J. Pratley, T. Haig, Allelopathy: from concept to reality, pp. 563–566, in D. L. Michalk and J. E. Pratley (eds.). Proceedings, 9th Australian Agronomy Conference, Wagga, Australia (1998).
- A. R. Putman, W. D. Duke, Science, 185, 370 (1974).
- P. K. Fay, W. B. Duke, Weed Sci. 25, 224, (1977).
- R. H. Dilday, J. Lin, W. Yan, Aust. J. Exp. Agr. 34, 907, (1994).
- M. Olofsdotter, D. Navarez, K. Moody, Ann. Appl. Biol. 127, 543, (1995).
- D. L. Liu D, J. V. Lovett , J. Chem. Ecol. 19, 2217, (1993).
- . J. Corcuera, Phytochem. 33, 741. (1993).
- I. Bouhaouel, A. Gfeller, M-L, Fauconnier, S. Rezgui, H. Slim, P. Jardin, BioControl 60, 425, (2015).
- R. Farhoudi, H-S- Zangane, S. Saeedipour, Res.Crop. 13, 467, (2012).
- I. Vasilakoglou, K. Dhima, A. Lithourgidis, Allelopathy Journal, 24, 309, (2009).
- R. Farhoudi, D. J. Lee, Plant. Natl.Sci.Ind.B 83, 447, (2013).
- S. Lebecque, J. m. Crowet, L. Lins B. M. Delory, P- du Jardin, M. L. Fauconnier, M. Deleu, Sci Rep. 8(1), 9784, (2018).
- M. Ben-Hammouda, H. Ghorbal, R. J. Kremer, O, Oueslati, OAgronomie 21: 65, (2001).
- N-O Bertholdsson, Weed ress 44, 78, (2004).
- N.Katerji, J.W.vanHoorn, A. Hamdy, M. Mastrorilli, C. Fares, S. Ceccarelli, S. Grando T.Oweis , Agricultural Water Management ,84, 184, (2006).
- A. Rehman, A. Ali Chandio, H. Imran, Uan Jingdong , Journal of the Saudi Society of Agricultural Sciences , 18, 269, (2019).
- H. Bravo, S. Copaja, M. Lamborot, Phytotoxicity of Phenolic Acids From Cereals». In Herbicides - Advances in Research, ed. Andrew Price. In Tech. (2013).
- S. V. Copaja, E. Villaroel, H. Bravo, L. Pizarro, V. Argandoña, Journal of Biosciences 61, 670, (2006).
- M. Schulz, A. Marocco, V. Tabaglio, f. Macias, J. Molinillo, Journal of Chemical Ecology 39, 154, (2013).
- D. Sicker, M. Frey, M. Schulz A. Gierl, International review of cytology 198, 319, (2000).
- H. Wu, T. Haig, J. Pratley, D. Lemerle, M. An, Journal of Chemical Ecology, 27, 125, (2001).
- A. Baghestani, C. Lemieux, G. Leroux, R. Baziramakenga, R. Simar, Weed Science 47, 498, (1999).
- W. Guenzi, T. McCalla, Agronomy Journal 58, 303, (1966).
- H. Bravo, M. Iglesias, S. Copaja, V. Argandoña, Revista latinoamericana de Química 38, 123, (2010).
- L. Overland, American Journal of Botany 53, 423, (1966).
- Y. Ishikawa, T. Kanke, Applied Entomology and Zoology 35, 251, (2000).
- B. Pastuszewska, S. Smulikowska, J. Wasilewko, L. Buraczewska, A. Ochtabinska, A. Mieczkowska, R. Lechowski, W. Bielecki, Archiv für Tierernaehrung.55, 1, (2001).
- H. Bravo, E. Clavijo, B. Weiss, Boletín de la Sociedad Chilena de Química, 46, 257, (2001).
- R. Hagin, Journal of Agricultural and Food Chemistry 37, 1143, (1989).
- H. Matsuo, K. Taniguchi, T. Hiramoto, T. Yamada, Y. Ichinose, K. Toyoda, K., Takeda, T. Shiraishi, Plant and Cell Physiology 42, 1103, (2001).
- A. Sadzawka, M. A. Carrasco, R. Grez, M. L. Mora, H. Flores, A. Neuman, Métodos de Análisis de Suelos. Instituto de Investigaciones Agropecuarias, (INIA). Serie Actas INIA 34, 59, 2006.
- OECD. Guidelines for testing of chemicals, Section 1 (106):Adsorption-
- Desorption using batch equilibrium method in soils. Environmental Health
- and Safety Division, Organisation for Economic Co-operation and
- Development (OECD), Environment. Directorate, Paris, France. 2000.
- X. Chen, Information 6, 14, (2015).
- M. R. Shariff, International Journal of Engineering Research and Development 1, 55, (2012).
- K. S. Ahmad, N. Rashid, M. F. Nazar, S. Tazaiyen, Journal of the Chemical Society of Pakistan 34-35, 1017, (2014).
- T. Garrido, C. Costa, J. Fraile, E. Orejudo, M. Niñerola, A. Ginebreda, L. Olivella, M. Figueras, Marine Pollution Bulletin, 26, 11, 613, (1998).
- Anaya, A. 2000 «Ecología Química». Ed. Plaza y Valdés. México, D.F. 1 ed. 255-292 (2000).
- N. Maqbool, A. Wahid, M. Farooq, Z. Cheema, K. Siddique, Allelopathy and Abiotic Stress Interaction in Crop Plants. En Allelopathy, Berlin, Heidelberg: Springer Berlin Heidelberg, 451-68, (2013).
- F. Muhammad, A. Bajwa, S. Cheema, Z. Cheema, Int. J. Agric. Biol. 15, 1367, (2013).
- H. Bravo, S. Copaja, Agricultural Sciences, 09, 1457, (2018)..
- A. Finizio, M. Vighi, D. Sandroni, Chemosphere 34, 131, (1997).
- C. Hansch, T. Fujita, J. Am. Chem. 86, 1616, (1964).
- A. Leo., C. Hansch, D. Elkins, Chemical Reviews 71, 525, (1971).
- S. Han, J. Qiao, Y. Zhang, L. Yang, H. Lian, X. Ge, H. Chen, Chemosphere 83, 131, (2011).
- J. Platts, S. Oldfield, M. Reif, A. Palmucci, E. Gabano, D. Osella, Biochemistry 100, 1199, (2006).
- SAG - Servicio agrícola y ganadero de Chile. 2012. Informe de ventas de plaguicidas de uso agrícola en Chile, año 2012.
- R. Alfaro, R. 2013. «Herbicidas asociados al caña de azúcar y su Potencial de Contaminación en el medio Ambiente». Liga agrícola industrial de la caña de azúcar LAICA: 1-63 (2013).
- Inderjit. 2005. «Soil Microorganisms: An Important Determinant of Allelopathic Activity». Plant and Soil 274, 227, (2005).
- S. Jilani Mahmood, A. Chaudhry, I. Hassan, M. Akram, A review». Annals of Microbiology 58, 351, (2008).
- U. Blum, S. Shafer, M. Lehman, Critical Reviews in Plant Sciences 18, 673, (1999).
- H. Flint, K. Scott, S. Duncan, P. Louis, E. Forano, Gut Microbes 3, 289, (2012).
- INECC – Instituto Nacional de Ecología y Cambio Climático. Características fisco-químicas de los plaguicidas y su transporte en el ambiente. México (2011).
- R. Rodríguez, R. Linares, E. Guadalupe, Revista del Instituto de investigaciones FIGMMG 12, 108, (2009).
- F. Islam, J. Wang, M. Farooq, M. Khan, L. Xu, J. Zhu, S. Muñoz, Q. Li, W. Zhou, Potential impact of the herbicide 2,4-D on human an ecosystems. Environment international (2017).
- F. Weber, J. Leclerc, Geology and genesis of the Moanda manganese deposits, Republic of Gabon. Geology and geochemistry of Manganese 2, 89, (1980).
- G. Castillo, ed. Ensayos Toxicológicos y Métodos de Evaluación de Calidad de Aguas. Estandarización, Intercalibración, Resultados y Aplicaciones. México: Instituto Mexicano de Tecnología del Agua. 100-189 (2004).