THIONE-THIOL TAUTOMERIC EQUILIBRIUM IN A DIHYDROPYRIMIDINE-THIONE: X RAY DIFFRACTION HELPED BY NMR, FTIR AND THEORETICAL CALCULATIONS

- tautomeric equilibrium,
- X-ray diffraction,
- Thione-thiol
Copyright (c) 2023 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
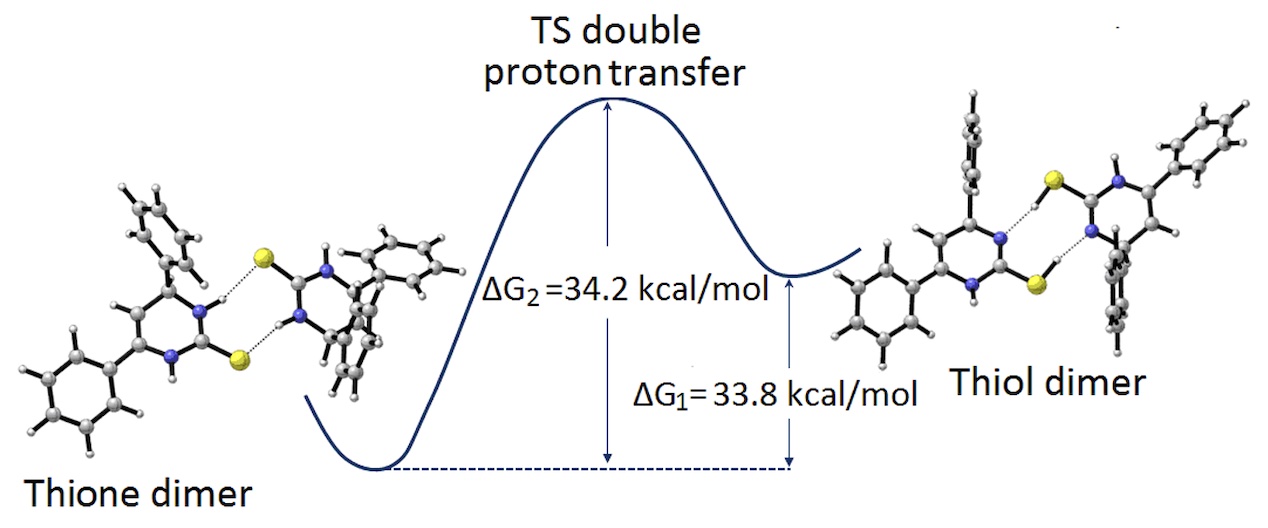
The solid state thione-thiol equilibrium in 4,6-diphenyl-3,4-dihydropyrimidine-2(1H)-thione (C16H14N2S) is analyzed through three different techniques, viz: Single Cristal X-Ray Diffraction (SCXRD), Nuclear Magnetic Resonance (NMR) and Fourier Transformed Infra-Red spectroscopy (FTIR), each one providing complementary information to the solution of the problem. The existence of thione-thiol equilibrium is firmly established, both in solution (by HNMR techniques) as in the solid state (by FTIR methods), and even if no traces of the thiol form could be found via SCXRD, some hints about the way in which the coexistence of both forms could be structurally achieved are provided by the structural analysis. In order to confirm the existence of this equilibrium, theoretical calculations were carried out at the B3LYP/6-311G(d,p) level theory, and a double proton transfer reaction is proposed.
References
- Frisch, M. J., et al. (2009). GAUSSIAN09. Gaussian Inc., Wallingford, CT, USA. http://www.gaussian.com.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179.
- Gupta, P. & Gupta, J. (2015). Chem Sci J. 6, 2-12.
- Kachroo, M., Panda, R. & Yadav, Y. (2014). Der Pharma Chemica, 6, 352-359.
- Kong, K. H., Chen, Y., Ma, X., Chui, W. K. & Lam, Y. (2004). J. Comb. Chem. 6, 928-933.
- Özdemir, N. & Türkpençe, D. (2013). Computational and Theoretical Chemistry 1025, 35–45.
- Bruker (2002). SAINT-NT V6.22a (Including SADABS). Data Reduction Software. Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- Bruker (2001). SMART-NT V5.624. Data Collection Software. Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122.
- Sheldrick, G. M. (2015). Acta Cryst., C71, 8–15.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155.
- Stoyanov, S., Petkov, I., Antonov, L., Stoyanova, T., Karagiannidis, P. & Aslanidis, P. (1990). Canadian Journal of Chemistry, 68, 1482-1489. 7.
- Trivedi, M. K., Branton, A., Trivedi, D., Shettigar, H., Bairwa, K. & Jana, S. (2015). Nat Prod Chem Res, 3:186.
- Zeng, R.-S., Zou, J.-P., Zhi, S.-J., Chen, J. & Shen, Q. (2003). Organic Letters, 5, 1657-1659.
- Zhang, L., Peng, X.-M., Damu, G. L. V., Geng, R.-X. & Zhou, C.-H. (2014). Medicinal Research Reviews, 34, 340-437.

