REMOVAL OF AMOXICILLIN VIA DIFFERENT METHODS, EMPHASIZING REMOVAL BY BIOPOLYMERS AND ITS DERIVATIVES. AN OVERVIEW.

- Antibiotics,
- Amoxicillin,
- Polymers,
- Removal,
- Bioadsorbents
Copyright (c) 2022 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Although pharmaceutical compounds such as antibiotics have been of great help to animals and humans, the excessive use of them have become a global problem due to the resistance of pathogens to these drugs, for this reason a series of methods have been reported that we will see below that allow to remove efficiently, economically, and environmentally friendly compounds such as antibiotics.
The aim of this overview is the removal of amoxicillin via different methods, emphasizing removal by biopolymers and its derivatives.
References
- Ghalkhani M, Zare N, Karimi F, Karaman C, Alizadeh M, Vasseghian Y. Recent advances in Ponceau dyes monitoring as food colorant substances by electrochemical sensors and developed procedures for their removal from real samples. Food and Chemical Toxicology. 2022:112830.
- Karimi-Maleh H, Khataee A, Karimi F, Baghayeri M, Fu L, Rouhi J, et al. A green and sensitive guanine-based DNA biosensor for idarubicin anticancer monitoring in biological samples: A simple and fast strategy for control of health quality in chemotherapy procedure confirmed by docking investigation. Chemosphere. 2022; 291:132928.
- Karaman C, Karaman O, Show P-L, Karimi-Maleh H, Zare N. Congo red dye removal from aqueous environment by cationic surfactant modified-biomass derived carbon: equilibrium, kinetic, and thermodynamic modeling, and forecasting via artificial neural network approach. Chemosphere. 2022; 290:133346.
- Kang BR, Kim SY, Kang M, Lee TK. Removal of pharmaceuticals and personal care products using native fungal enzymes extracted during the ligninolytic process. Environmental Research. 2021; 195:110878.
- Nava-Andrade K, Carbajal-Arízaga G, Obregón S, Rodríguez-González V. Layered double hydroxides and related hybrid materials for removal of pharmaceutical pollutants from water. Journal of Environmental Management. 2021; 288:112399.
- Yayayürük O, Yayayürük AE, Özmen P, Karagöz B. PDMAEMA grafted microspheres as an efficient adsorbent for the removal of Sunset yellow from pharmaceutical preparations, beverages and waste water. European Polymer Journal. 2020; 141:110089.
- Olasupo A, Suah FBM. Recent advances in the removal of pharmaceuticals and endocrine-disrupting compounds in the aquatic system: A case of polymer inclusion membranes. Journal of hazardous materials. 2021; 406:124317.
- Moradi O, Alizadeh H, Sedaghat S. Removal of pharmaceuticals (diclofenac and amoxicillin) by maltodextrin/reduced graphene and maltodextrin/reduced graphene/copper oxide nanocomposites. Chemosphere. 2022; 299:134435.
- Ricky R, Chiampo F, Shanthakumar S. Efficacy of Ciprofloxacin and Amoxicillin Removal and the Effect on the Biochemical Composition of Chlorella vulgaris. Bioengineering. 2022; 9(4):134.
- Danner M-C, Robertson A, Behrends V, Reiss J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Science of The Total Environment. 2019;664:793-804.
- Singer AC, Shaw H, Rhodes V, Hart A. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Frontiers in microbiology. 2016; 7:1728.
- Cabello FC. Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environmental microbiology. 2006; 8(7):1137-44.
- Kümmerer K. Resistance in the environment. Journal of antimicrobial chemotherapy. 2004; 54(2):311-20.
- Gorito AM, Ribeiro ARL, Rodrigues P, Pereira MFR, Guimarães L, Almeida CMR, et al. Antibiotics removal from aquaculture effluents by ozonation: chemical and toxicity descriptors. Water Research. 2022;218:118497.
- You X, Li H, Pan B, You M, Sun W. Interactions between antibiotics and heavy metals determine their combined toxicity to Synechocystis sp. Journal of Hazardous Materials. 2022;424:127707.
- Luan Y, Chen K, Zhao J, Cheng L. Comparative Study on Synergistic Toxicity of Enrofloxacin Combined with Three Antibiotics on Proliferation of THLE-2 Cell. Antibiotics. 2022;11(3):394.
- Editors PM. Antimicrobial resistance: is the world UNprepared? : Public Library of Science San Francisco, CA USA; 2016. p. e1002130.
- Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 2022;399(10325):629-55.
- Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. The Lancet infectious diseases. 2019;19(1):56-66.
- Chavarria-Pizarro T, Resl P, Kuhl-Nagel T, Janjic A, Fernandez Mendoza F, Werth S. Antibiotic-Induced Treatments Reveal Stress-Responsive Gene Expression in the Endangered Lichen Lobaria pulmonaria. Journal of Fungi. 2022;8(6):625.
- Kümmerer K. Antibiotics in the aquatic environment–a review–part I. Chemosphere. 2009;75(4):417-34.
- Kümmerer K. Antibiotics in the aquatic environment–a review–part II. Chemosphere. 2009;75(4):435-41.
- Mojica ERE, Aga DS. Antibiotics Pollution in Soil and Water: Potential Ecological and Human Health Issues. In: Nriagu JO, editor. Encyclopedia of Environmental Health. Burlington: Elsevier; 2011. p. 97-110.
- Zhang R, Yang S, An Y, Wang Y, Lei Y, Song L. Antibiotics and antibiotic resistance genes in landfills: A review. Science of The Total Environment. 2022;806:150647.
- Katz L, Baltz RH. Natural product discovery: past, present, and future. Journal of Industrial Microbiology and Biotechnology. 2016;43(2-3):155-76.
- Hutchings MI, Truman AW, Wilkinson B. Antibiotics: past, present and future. Current Opinion in Microbiology. 2019;51:72-80.
- Prescott JF. The resistance tsunami, antimicrobial stewardship, and the golden age of microbiology. Veterinary microbiology. 2014;171(3-4):273-8.
- Kümmerer K. Significance of antibiotics in the environment. Journal of Antimicrobial Chemotherapy. 2003;52(1):5-7.
- Laxminarayan R, Chaudhury RR. Antibiotic resistance in India: drivers and opportunities for action. PLoS medicine. 2016;13(3):e1001974.
- Neil J. Report on Antimicrobial Resistance. Report on Antimicrobial Resistance. 2016.
- Jiang Q, Feng M, Ye C, Yu X. Effects and relevant mechanisms of non-antibiotic factors on the horizontal transfer of antibiotic resistance genes in water environments: A review. Science of The Total Environment. 2022;806:150568.
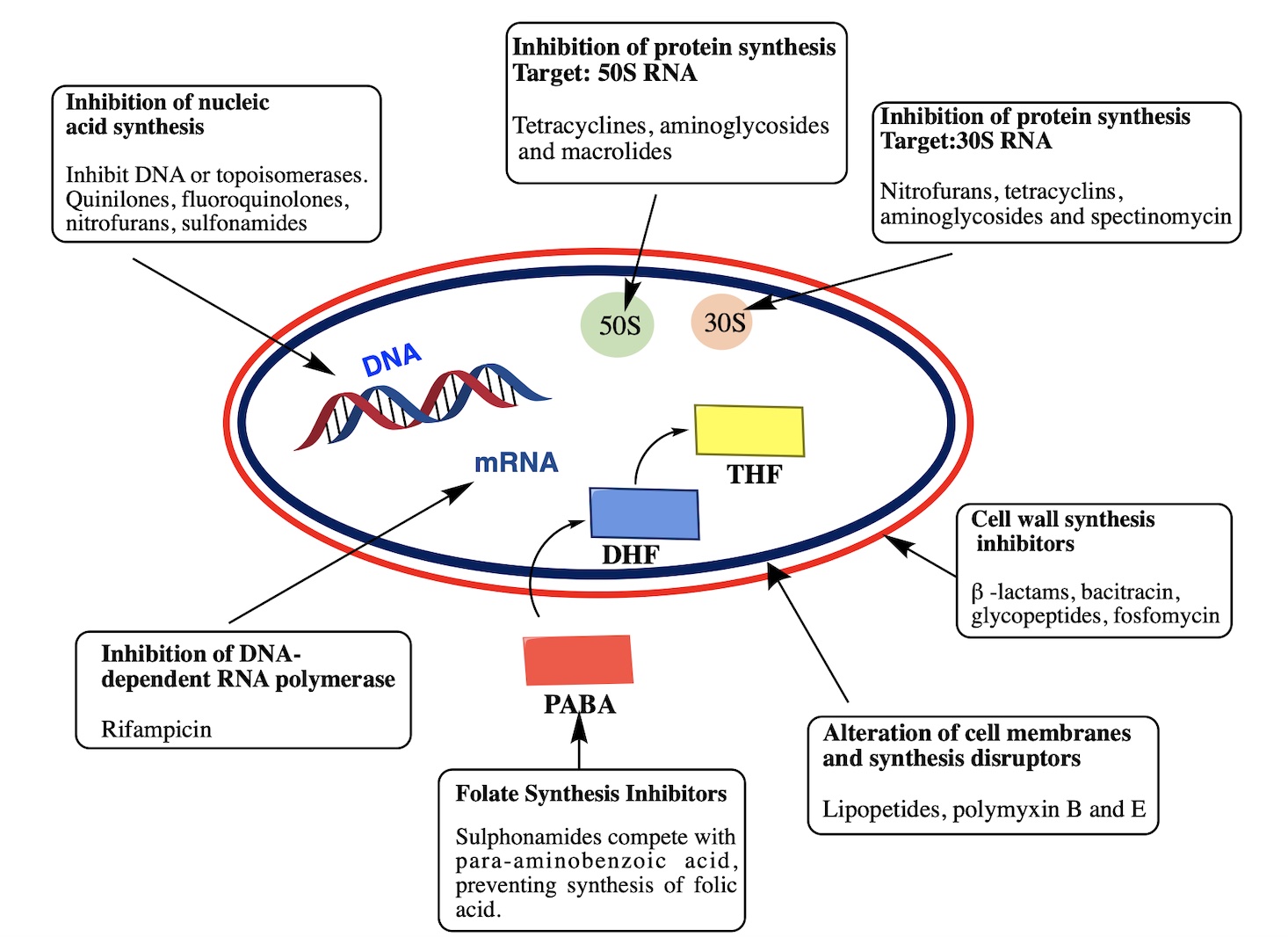
- O’Rourke A, Beyhan S, Choi Y, Morales P, Chan AP, Espinoza JL, et al. Mechanism-of-Action Classification of Antibiotics by Global Transcriptome Profiling. Antimicrobial Agents and Chemotherapy. 2020;64(3):e01207-19.
- Etebu E, Arikekpar I. Antibiotics: Classification and mechanisms of action with emphasis on molecular perspectives. Int J Appl Microbiol Biotechnol Res. 2016;4(2016):90-101.
- Dousa KM, Nguyen DC, Kurz SG, Taracila MA, Bethel CR, Schinabeck W, et al. Inhibiting Mycobacterium abscessus Cell Wall Synthesis: Using a Novel Diazabicyclooctane β-Lactamase Inhibitor To Augment β-Lactam Action. Mbio. 2022;13(1):e03529-21.
- Crowe-McAuliffe C, Wilson DN. Putting the antibiotics chloramphenicol and linezolid into context. Nature Structural & Molecular Biology. 2022;29(2):79-81.
- Kümmerer K. Antibiotics in the aquatic environment – A review – Part I. Chemosphere. 2009;75(4):417-34.
- Krause KM, Serio AW, Kane TR, Connolly LE. Aminoglycosides: An Overview. Cold Spring Harb Perspect Med. 2016;6(6).
- Kotra LP, Haddad J, Mobashery S. Aminoglycosides: perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrobial agents and chemotherapy. 2000;44(12):3249-56.
- Stubbings W, Bostock J, Ingham E, Chopra I. Mechanisms of the post-antibiotic effects induced by rifampicin and gentamicin in Escherichia coli. Journal of Antimicrobial Chemotherapy. 2006;58(2):444-8.
- Könönen E, Wade WG. Actinomyces and Related Organisms in Human Infections. Clinical Microbiology Reviews. 2015;28(2):419-42.
- Koyuncu I, Arikan OA, Wiesner MR, Rice C. Removal of hormones and antibiotics by nanofiltration membranes. Journal of membrane science. 2008;309(1-2):94-101.
- Zhu Y, Hao W, Wang X, Ouyang J, Deng X, Yu H, et al. Antimicrobial peptides, conventional antibiotics, and their synergistic utility for the treatment of drug‐resistant infections. Medicinal Research Reviews. 2022.
- Sumbatyan N, Kuznetsova I, Karpenko V, Fedorova N, Chertkov V, Korshunova G, et al. Amino acid and peptide derivatives of the tylosin family of macrolide antibiotics modified by aldehyde function. Russian journal of bioorganic chemistry. 2010;36(2):245-56.
- Weiss RB, editor The anthracyclines: will we ever find a better doxorubicin? Seminars in oncology; 1992.
- Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, et al. Doxorubicin: the good, the bad and the ugly effect. Current medicinal chemistry. 2009;16(25):3267-85.
- Moutabian H, Ghahramani‐Asl R, Mortezazadeh T, Laripour R, Narmani A, Zamani H, et al. The cardioprotective effects of nano‐curcumin against doxorubicin‐induced cardiotoxicity: A systematic review. Biofactors. 2022.
- Hortobágyi GN. Anthracyclines in the Treatment of Cancer. Drugs. 1997;54(4):1-7.
- Gong J, Ji Y, Liu X, Zheng Y, Zhen Y. Mithramycin suppresses tumor growth by regulating CD47 and PD-L1 expression. Biochemical Pharmacology. 2022;197:114894.
- Schweer D, McCorkle JR, Rohr J, Tsodikov OV, Ueland F, Kolesar J. Mithramycin and Analogs for Overcoming Cisplatin Resistance in Ovarian Cancer. Biomedicines. 2021;9(1):70.
- Kormanec J, Novakova R, Csolleiova D, Feckova L, Rezuchova B, Sevcikova B, et al. The antitumor antibiotic mithramycin: new advanced approaches in modification and production. Applied Microbiology and Biotechnology. 2020;104(18):7701-21.
- Furman BL. Streptozotocin‐induced diabetic models in mice and rats. Current protocols in pharmacology. 2015;70(1):5.47. 1-5.. 20.
- Capdevila J, Ducreux M, Carbonero RG, Grande E, Halfdanarson T, Pavel M, et al. STREPTOZOTOCIN. 1982-2022: FORTY YEARS FROM THE FDA’S APPROVAL TO TREAT PANCREATIC NEUROENDOCRINE TUMORS. Neuroendocrinology. 2022.
- Wu C-Y, Liang C-H, Liang Z-C. Enhanced production of fruiting bodies and bioactive compounds of Cordyceps militaris with grain substrates and cultivation patterns. Journal of the Taiwan Institute of Chemical Engineers. 2022;132:104138.
- Suarez C, Gudiol F. Beta-lactam antibiotics. Enfermedades infecciosas y microbiologia clinica. 2009;27(2):116-29.
- Morin RB, Gorman M. The Biology of B-Lactam Antibiotics: Elsevier; 2014.
- Minaldi E, Phillips EJ, Norton A. Immediate and Delayed Hypersensitivity Reactions to Beta-Lactam Antibiotics. Clinical Reviews in Allergy & Immunology. 2022;62(3):449-62.
- Lobritz MA, Andrews IW, Braff D, Porter CB, Gutierrez A, Furuta Y, et al. Increased energy demand from anabolic-catabolic processes drives β-lactam antibiotic lethality. Cell Chemical Biology. 2022;29(2):276-86. e4.
- Palacio DA, Urbano BF, Rivas BL. Application of nanocomposite polyelectrolytes for the removal of antibiotics as emerging pollutants in water. Journal of Water Process Engineering. 2022;46:102582.
- Palacio DA, Urbano BF, Rivas BL. Water-soluble polymers with the ability to remove amoxicillin as emerging pollutant from water. Environmental Technology & Innovation. 2021;23:101589.
- Chandel AK, Rao LV, Narasu ML, Singh OV. The realm of penicillin G acylase in β-lactam antibiotics. Enzyme and Microbial Technology. 2008;42(3):199-207.
- Schneider P, Hawser S, Islam K. Iclaprim, a novel diaminopyrimidine with potent activity on trimethoprim sensitive and resistant bacteria. Bioorganic & medicinal chemistry letters. 2003;13(23):4217-21.
- Simo B, Perelló L, Ortiz R, Castineiras A, Latorre J, Canton E. Interactions of metal ions with a 2, 4-diaminopyrimidine derivative (trimethoprim): Antibacterial studies. Journal of inorganic biochemistry. 2000;81(4):275-83.
- Li X, Lu H, Ji M, Sun K, Pu F, Ding Y, et al. Synthesis and biological properties of maleimide-based macrocyclic lactone enediyne. Organic & Biomolecular Chemistry. 2022.
- Grissom JW, Gunawardena GU, Klingberg D, Huang D. The chemistry of enediynes, enyne allenes and related compounds. Tetrahedron. 1996;52(19):6453-518.
- Damle NK, Frost P. Antibody-targeted chemotherapy with immunoconjugates of calicheamicin. Current opinion in pharmacology. 2003;3(4):386-90.
- Goodin S, Kane MP, Rubin EH. Epothilones: mechanism of action and biologic activity. Journal of clinical oncology. 2004;22(10):2015-25.
- Zhang P, Zhang L, Jiang X, Diao X-t, Li S, Li D-d, et al. Docking-guided rational engineering of a macrolide glycosyltransferase glycodiversifies epothilone B. Communications biology. 2022;5(1):1-11.
- Acharya Y, Dhanda G, Sarkar P, Haldar J. Pursuit of next-generation glycopeptides: a journey with vancomycin. Chemical Communications. 2022;58(12):1881-97.
- Duncan LR, Wang W, Sader HS. In Vitro Potency and Spectrum of the Novel Polymyxin MRX-8 Tested against Clinical Isolates of Gram-Negative Bacteria. Antimicrobial Agents and Chemotherapy. 2022;66(5):e00139-22.
- Velkov T, Thompson PE, Nation RL, Li J. Structure− activity relationships of polymyxin antibiotics. Journal of medicinal chemistry. 2010;53(5):1898-916.
- Leclercq R. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clinical infectious diseases. 2002;34(4):482-92.
- Armengol Álvarez L, Van de Sijpe G, Desmet S, Metsemakers W-J, Spriet I, Allegaert K, et al. Ways to Improve Insights into Clindamycin Pharmacology and Pharmacokinetics Tailored to Practice. Antibiotics. 2022;11(5):701.
- Khoshnood S, Shirani M, Dalir A, Moradi M, Haddadi MH, Sadeghifard N, et al. Antiviral effects of azithromycin: A narrative review. Biomedicine & Pharmacotherapy. 2022;147:112682.
- Kocsmár É, Buzás GM, Szirtes I, Kocsmár I, Kramer Z, Szijártó A, et al. Primary and secondary clarithromycin resistance in Helicobacter pylori and mathematical modeling of the role of macrolides. Nature communications. 2021;12(1):1-12.
- Ashraf A, Liu G, Yousaf B, Arif M, Ahmed R, Rashid A, et al. Phyto-mediated photocatalysis: a critical review of in-depth base to reactive radical generation for erythromycin degradation. Environmental Science and Pollution Research. 2022:1-32.
- Bai J, Ding X, Mou H, Wang S, Chen S. Menthol in Combination with Iontophoresis Promotes Natamycin Penetration through the Cornea: In Vitro and In Vivo Studies. Bulletin of Experimental Biology and Medicine. 2022;172(3):318-23.
- Quirós LM, Carbajo RJ, Salas JA. Inversion of the anomeric configuration of the transferred sugar during inactivation of the macrolide antibiotic oleandomycin catalyzed by a macrolide glycosyltransferase. FEBS letters. 2000;476(3):186-9.
- Han J, Sharipov M, Hwang S, Lee Y, Huy BT, Lee Y-I. Water-stable perovskite-loaded nanogels containing antioxidant property for highly sensitive and selective detection of roxithromycin in animal-derived food products. Scientific Reports. 2022;12(1):1-11.
- Nasiri A, Mokhtari S, Jahani R, Daraie B, Yazdanpanah H, Faizi M, et al. Challenges for the determination of spiramycin in aqueous matrices using LC-MS/MS: evidence for the solvent intrusion on the molecule integrity. RSC advances. 2022;12(27):17096-103.
- Liu Z, Chen J, Zhao S, Pang Y, Shen X, Lei H, et al. Immunochromatographic assays based on three kinds of nanoparticles for the rapid and highly sensitive detection of tylosin and tilmicosin in eggs. Microchimica Acta. 2022;189(1):1-11.
- Pantcheva IN, Stambolyiska RD, Petkov NN, Tadjer AV, Simova SD, Stoyanova RK, et al. Mononuclear copper (II) complexes of the macrolide antibiotics tylosin and tilmicosin. Transition Metal Chemistry. 2022;47(1):67-76.
- Mitchell SM, Ullman JL, Teel AL, Watts RJ. Hydrolysis of amphenicol and macrolide antibiotics: Chloramphenicol, florfenicol, spiramycin, and tylosin. Chemosphere. 2015;134:504-11.
- Leng D, Sheng Y, Wang H, Wei J, Ou Y, Deng Z, et al. Determination of the Protein-Protein Interactions within Acyl Carrier Protein (MmcB)-Dependent Modifications in the Biosynthesis of Mitomycin. Molecules. 2021;26(22):6791.
- Jagannathan SV, Manemann EM, Rowe SE, Callender MC, Soto W. Marine actinomycetes, new sources of biotechnological products. Marine Drugs. 2021;19(7):365.
- Le VVH, Rakonjac J. Nitrofurans: revival of an “old” drug class in the fight against antibiotic resistance. PLoS Pathogens. 2021;17(7):e1009663.
- GOTSIRIDZE D, BARAMIDZE K, CHIKVILADZE T, OTARASHVILI T. NITROFURANS AND THEIR METABOLITES IN FOOD. EXPERIMENTAL & CLINICAL MEDICINE GEORGIA. 2022(4).
- Bourque DL, Neumayr A, Libman M, Chen LH. Treatment strategies for nitroimidazole-refractory giardiasis: a systematic review. Journal of Travel Medicine. 2022;29(1):taab120.
- Gupta R, Sharma S, Singh R, Vishwakarma RA, Mignani S, Singh PP. Functionalized Nitroimidazole Scaffold Construction and Their Pharmaceutical Applications: A 1950–2021 Comprehensive Overview. Pharmaceuticals. 2022;15(5):561.
- Benyas D, Sobel JD. Mixed vaginitis due to bacterial vaginosis and candidiasis. Journal of Lower Genital Tract Disease. 2022;26(1):68-70.
- Yang L, Zeng L, Tao Y, Wang D, Zhang K, Tian M, et al. Galli Gigerii Endothelium Corneum derived fluorescent carbon dots and their application as sensing platform for nitroimidazoles and cell imaging. Microchemical Journal. 2022;174:107089.
- Feder HMJ, Osier C, Maderazo EG. Chloramphenicol: a review of its use in clinical practice. Reviews of infectious diseases. 1981;3(3):479-91.
- Wei CF, Chang SK, Shien JH, Kuo HC, Chen WY, Chou CC. Synergism between two amphenicol of antibiotics, florfenicol and thiamphenicol, against Staphylococcus aureus. Veterinary Record. 2016;178(13):319-.
- Lin J, Nishiyama M, Kuzuyama T. Identification of the biosynthetic gene cluster for the herbicide phosphonothrixin in Saccharothrix sp. ST-888. The Journal of Antibiotics. 2015;68(5):357-9.
- Zhang Y, Chen L, Wilson JA, Cui J, Roodhouse H, Kayrouz C, et al. Valinophos Reveals a New Route in Microbial Phosphonate Biosynthesis That Is Broadly Conserved in Nature. Journal of the American Chemical Society. 2022;144(22):9938-48.
- Horsman GP, Zechel DL. Phosphonate biochemistry. Chemical reviews. 2017;117(8):5704-83.
- Antoszczak M, Steverding D, Huczyński A. Anti-parasitic activity of polyether ionophores. European journal of medicinal chemistry. 2019;166:32-47.
- Kochansky J, Pettis J. Screening additional antibiotics for efficacy against American foulbrood. Journal of apicultural research. 2005;44(1):24-8.
- MichałAntoszczak JR, Huczynski A. Structure and biological activity of polyether ionophores and their semisynthetic derivatives. Bioactive Natural Products: Chemistry and Biology. 2015.
- Kevin Ii DA, Meujo DA, Hamann MT. Polyether ionophores: broad-spectrum and promising biologically active molecules for the control of drug-resistant bacteria and parasites. Expert opinion on drug discovery. 2009;4(2):109-46.
- Huet A-C, Charlier C, Tittlemier SA, Singh G, Benrejeb S, Delahaut P. Simultaneous determination of (fluoro) quinolone antibiotics in kidney, marine products, eggs, and muscle by enzyme-linked immunosorbent assay (ELISA). Journal of agricultural and food chemistry. 2006;54(8):2822-7.
- Rose MD, Bygrave J, Stubbings GW. Extension of multi-residue methodology to include the determination of quinolones in food. Analyst. 1998;123(12):2789-96.
- van Vyncht G, Jànosi A, Bordin G, Toussaint B, Maghuin-Rogister G, De Pauw E, et al. Multiresidue determination of (fluoro) quinolone antibiotics in swine kidney using liquid chromatography–tandem mass spectrometry. Journal of Chromatography A. 2002;952(1-2):121-9.
- Cañada‐Cañada F, Espinosa‐Mansilla A, Muñoz de la Peña A. Separation of fifteen quinolones by high performance liquid chromatography: Application to pharmaceuticals and ofloxacin determination in urine. Journal of separation science. 2007;30(9):1242-9.
- Li Y, Hao X, Ji B, Xu C, Chen W, Shen C, et al. Rapid determination of 19 quinolone residues in spiked fish and pig muscle by high-performance liquid chromatography (HPLC) tandem mass spectrometry. Food Additives & Contaminants: Part A. 2009;26(3):306-13.
- Sharma SK, Sharma A, Kadhiravan T, Tharyan P. Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV‐negative people at risk of active TB. Evidence‐Based Child Health: A Cochrane Review Journal. 2014;9(1):169-294.
- Bolleddula J, Gopalakrishnan S, Hu P, Dong J, Venkatakrishnan K. Alternatives to rifampicin: A review and perspectives on the choice of strong CYP3A inducers for clinical drug‐drug interaction studies. Clinical and Translational Science. 2022.
- Organization WH. Technical report on critical concentrations for drug susceptibility testing of isoniazid and the rifamycins (rifampicin, rifabutin and rifapentine): World Health Organization; 2021.
- Riviere JE, Craigmill AL, Sundlof SF. Sulfonamides. Handbook of Comparative Pharmacokinetics and Residues of Veterinary Antimicrobials: CRC Press; 2018. p. 339-416.
- Finegold SM, Ziment I. Sulfonamides, nitrofurans and nalidixic acid. Pediatric Clinics of North America. 1968;15(1):95-105.
- Suo D, Wang P, Xiao Z, Zhang S, Zhuang H, Li Y, et al. Multiresidue determination of 27 sulfonamides in poultry feathers and its application to a sulfamethazine pharmacokinetics study on laying hen feathers and sulfonamide residue monitoring on poultry feathers. Journal of agricultural and food chemistry. 2019;67(40):11236-43.
- Cheng S, Wei Z, Zhiming X, Yang L, Xia F. Trace analysis and identification of 33 sulfonamides and sulfonamide potentiators in eggs by ultrahigh-performance liquid chromatography coupled with quadrupole-high-field orbitrap high-resolution mass spectrometry. Analytical Methods. 2021;13(38):4452-60.
- Palacio DA, Leiton LM, Urbano BF, Rivas BL. Tetracycline removal by polyelectrolyte copolymers in conjunction with ultrafiltration membranes through liquid-phase polymer-based retention. Environmental research. 2020;182:109014.
- Palacio DA, Becerra Y, Urbano BF, Rivas BL. Antibiotics removal using a chitosan-based polyelectrolyte in conjunction with ultrafiltration membranes. Chemosphere. 2020;258:127416.
- Rivas BL, Oñate P, Palacio DA. Removal of oxytetracycline by polymers. an overview. Journal of the Chilean Chemical Society. 2020;65(4):4943-7.
- Urbano BF, Bustamante S, Palacio DA, Vera M, Rivas BL. Polymer supports for the removal and degradation of hazardous organic pollutants: an overview. Polymer International. 2020;69(4):333-45.
- Nelson ML, Levy SB. The history of the tetracyclines. Annals of the New York Academy of Sciences. 2011;1241(1):17-32.
- Nadeem SF, Gohar UF, Tahir SF, Mukhtar H, Pornpukdeewattana S, Nukthamna P, et al. Antimicrobial resistance: more than 70 years of war between humans and bacteria. Critical Reviews in Microbiology. 2020;46(5):578-99.
- Waglechner N, Wright G. Antibiotic resistance: It's bad, but why isn't it worse? BMC Biology. 2017;15.
- Zhang Q-Q, Ying G-G, Pan C-G, Liu Y-S, Zhao J-L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environmental science & technology. 2015;49(11):6772-82.
- Zhang T, Yang Y, Pruden A. Effect of temperature on removal of antibiotic resistance genes by anaerobic digestion of activated sludge revealed by metagenomic approach. Applied microbiology and biotechnology. 2015;99(18):7771-9.
- Yang Y, Song W, Lin H, Wang W, Du L, Xing W. Antibiotics and antibiotic resistance genes in global lakes: a review and meta-analysis. Environment international. 2018;116:60-73.
- Ashiq A, Walpita J, Vithanage M. Functionalizing non-smectic clay via methoxy-modification for enhanced removal and recovery of oxytetracycline from aqueous media. Chemosphere. 2021;276:130079.
- Zhang L, Song X, Liu X, Yang L, Pan F, Lv J. Studies on the removal of tetracycline by multi-walled carbon nanotubes. Chemical engineering journal. 2011;178:26-33.
- Grenni P, Ancona V, Barra Caracciolo A. Ecological effects of antibiotics on natural ecosystems: A review. Microchemical Journal. 2018;136:25-39.
- Anastopoulos I, Pashalidis I, Orfanos AG, Manariotis ID, Tatarchuk T, Sellaoui L, et al. Removal of caffeine, nicotine and amoxicillin from (waste) waters by various adsorbents. A review. Journal of Environmental Management. 2020;261:110236.
- Sanseverino I, Navarro A, Loos R, Marinov D, Lettieri T. State of the Art on the Contribution of Water to Antimicrobial Resistance2018.
- Kapoor G, Saigal S, Elongavan A. Action and resistance mechanisms of antibiotics: A guide for clinicians. Journal of Anaesthesiology Clinical Pharmacology. 2017;33:300.
- Geissen V, Mol H, Klumpp E, Umlauf G, Nadal M, Van der Ploeg M, et al. Emerging pollutants in the environment: a challenge for water resource management. International soil and water conservation research. 2015;3(1):57-65.
- Cuerda-Correa EM, Alexandre-Franco MF, Fernández-González C. Advanced oxidation processes for the removal of antibiotics from water. An overview. Water. 2020;12(1):102.
- Adzitey F. Antibiotic classes and antibiotic susceptibility of bacterial isolates from selected poultry; a mini review. 2015.
- Dincer S, Yigittekin ES. Spreading of antibiotic resistance with wastewater. Biological Wastewater Treatment and Resource Recovery. 2017;73.
- Hernández F, Calısto-Ulloa N, Gómez-Fuentes C, Gómez M, Ferrer J, González-Rocha G, et al. Occurrence of antibiotics and bacterial resistance in wastewater and sea water from the Antarctic. Journal of hazardous materials. 2019;363:447-56.
- Zhuang M, Achmon Y, Cao Y, Liang X, Chen L, Wang H, et al. Distribution of antibiotic resistance genes in the environment. Environmental Pollution. 2021:117402.
- Begum S, Begum T, Rahman N, Khan RA. A review on antibiotic resistance and way of combating antimicrobial resistance. GSC Biological and Pharmaceutical Sciences. 2021;14(2):087-97.
- Bradford PA. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clinical microbiology reviews. 2001;14(4):933-51.
- Hooper DC. Emerging mechanisms of fluoroquinolone resistance. Emerging infectious diseases. 2001;7(2):337.
- Roberts MC, Sutcliffe J, Courvalin P, Jensen LB, Rood J, Seppala H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrobial agents and chemotherapy. 1999;43(12):2823-30.
- Hakenbeck R, Balmelle N, Weber B, Gardès C, Keck W, de Saizieu A. Mosaic genes and mosaic chromosomes: intra-and interspecies genomic variation of Streptococcus pneumoniae. Infection and Immunity. 2001;69(4):2477-86.
- Markham PN, Neyfakh AA. Efflux-mediated drug resistance in Gram-positive bacteria. Current opinion in microbiology. 2001;4(5):509-14.
- Poole K. Multidrug resistance in Gram-negative bacteria. Current opinion in microbiology. 2001;4(5):500-8.
- Douša M, Hosmanová R. Rapid determination of amoxicillin in premixes by HPLC. Journal of pharmaceutical and biomedical analysis. 2005;37(2):373-7.
- Hirte K, Seiwert B, Schüürmann G, Reemtsma T. New hydrolysis products of the beta-lactam antibiotic amoxicillin, their pH-dependent formation and search in municipal wastewater. Water research. 2016;88:880-8.
- Geddes AM, Klugman KP, Rolinson GN. Introduction: historical perspective and development of amoxicillin/clavulanate. International Journal of Antimicrobial Agents. 2007;30:109-12.
- Kotwani A, Holloway K. Trends in antibiotic use among outpatients in New Delhi, India. BMC infectious diseases. 2011;11(1):1-9.
- Marlière G, Ferraz M, dos Santos JQ. Antibiotic consumption patterns and drug leftovers in 6000 Brazilian households. Advances in therapy. 2000;17(1):32-44.
- Arancibia A, Guttmann J, Gonzalez G, Gonzalez C. Absorption and disposition kinetics of amoxicillin in normal human subjects. Antimicrobial agents and chemotherapy. 1980;17(2):199-202.
- Gordon RC, Regamey C, Kirby WM. Comparative clinical pharmacology of amoxicillin and ampicillin administered orally. Antimicrobial agents and chemotherapy. 1972;1(6):504-7.
- Andreozzi R, Canterino M, Marotta R, Paxeus N. Antibiotic removal from wastewaters: the ozonation of amoxicillin. Journal of hazardous Materials. 2005;122(3):243-50.
- Mutiyar PK, Mittal AK. Occurrences and fate of an antibiotic amoxicillin in extended aeration-based sewage treatment plant in Delhi, India: a case study of emerging pollutant. Desalination and Water Treatment. 2013;51(31-33):6158-64.
- Aydin E, Talinli I. Analysis, occurrence and fate of commonly used pharmaceuticals and hormones in the Buyukcekmece Watershed, Turkey. Chemosphere. 2013;90(6):2004-12.
- Foti C, Giuffrè O. Interaction of Ampicillin and Amoxicillin with Mn2+: A Speciation Study in Aqueous Solution. Molecules. 2020;25(14):3110.
- Proctor P, Gensmantel NP, Page MI. The chemical reactivity of penicillins and other β-lactam antibiotics. Journal of the Chemical Society, Perkin Transactions 2. 1982(9):1185-92.
- Hugonnet JE, Mengin-Lecreulx D, Monton A, den Blaauwen T, Carbonnelle E, Veckerlé C, et al. Factors essential for L,D-transpeptidase-mediated peptidoglycan cross-linking and β-lactam resistance in Escherichia coli. Elife. 2016;5.
- Erah P, Goddard A, Barrett D, Shaw P, Spiller R. The stability of amoxycillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. The Journal of antimicrobial chemotherapy. 1997;39(1):5-12.
- Dimitrakopoulou D, Rethemiotaki I, Frontistis Z, Xekoukoulotakis NP, Venieri D, Mantzavinos D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. Journal of Environmental Management. 2012;98:168-74.
- Elmolla ES, Chaudhuri M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination. 2010;252(1-3):46-52.
- Çağlar Yılmaz H, Akgeyik E, Bougarrani S, El Azzouzi M, Erdemoğlu S. Photocatalytic degradation of amoxicillin using Co-doped TiO2 synthesized by reflux method and monitoring of degradation products by LC–MS/MS. Journal of Dispersion Science and Technology. 2020;41(3):414-25.
- Reyns T, Cherlet M, De Baere S, De Backer P, Croubels S. Rapid method for the quantification of amoxicillin and its major metabolites in pig tissues by liquid chromatography-tandem mass spectrometry with emphasis on stability issues. Journal of Chromatography B. 2008;861(1):108-16.
- De Baere S, Wassink P, Croubels S, De Boever S, Baert K, De Backer P. Quantitative liquid chromatographic–mass spectrometric analysis of amoxycillin in broiler edible tissues. Analytica chimica acta. 2005;529(1-2):221-7.
- Freitas A, Barbosa J, Ramos F. Determination of Amoxicillin Stability in Chicken Meat by Liquid Chromatography–Tandem Mass Spectrometry. Food Analytical Methods. 2012;5(3):471-9.
- Jerzsele Á, Nagy G. The stability of amoxicillin trihydrate and potassium clavulanate combination in aqueous solutions. Acta Veterinaria Hungarica. 2009;57(4):485-93.
- Pham TH, Bui HM, Bui TX. Advanced oxidation processes for the removal of pesticides. Current Developments in Biotechnology and Bioengineering: Elsevier; 2020. p. 309-30.
- Pham TD, Vu TN, Nguyen HL, Le PHP, Hoang TS. Adsorptive removal of antibiotic ciprofloxacin from aqueous solution using protein-modified nanosilica. Polymers. 2020;12(1):57.
- Guo C, Wang K, Hou S, Wan L, Lv J, Zhang Y, et al. H2O2 and/or TiO2 photocatalysis under UV irradiation for the removal of antibiotic resistant bacteria and their antibiotic resistance genes. Journal of hazardous materials. 2017;323:710-8.
- Michael I, Hapeshi E, Michael C, Varela A, Kyriakou S, Manaia C, et al. Solar photo-Fenton process on the abatement of antibiotics at a pilot scale: degradation kinetics, ecotoxicity and phytotoxicity assessment and removal of antibiotic resistant enterococci. Water research. 2012;46(17):5621-34.
- Kasprzyk-Hordern B, Ziółek M, Nawrocki J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Applied Catalysis B: Environmental. 2003;46(4):639-69.
- Kurt A, Mert BK, Özengin N, Sivrioğlu Ö, Yonar T. Treatment of antibiotics in wastewater using advanced oxidation processes (AOPs). Physico-Chemical Wastewater Treatment and Resource Recovery. 2017;175.
- Gerrity D, Gamage S, Holady JC, Mawhinney DB, Quiñones O, Trenholm RA, et al. Pilot-scale evaluation of ozone and biological activated carbon for trace organic contaminant mitigation and disinfection. Water research. 2011;45(5):2155-65.
- De la Cruz N, Giménez J, Esplugas S, Grandjean D, De Alencastro L, Pulgarin C. Degradation of 32 emergent contaminants by UV and neutral photo-fenton in domestic wastewater effluent previously treated by activated sludge. Water research. 2012;46(6):1947-57.
- Saharan VK, Pinjari DV, Gogate PR, Pandit AB. Advanced oxidation technologies for wastewater treatment: an overview: Elsevier, Butterworth, Heinemann, UK; 2014.
- Sgroi M, Anumol T, Vagliasindi FG, Snyder SA, Roccaro P. Comparison of the new Cl2/O3/UV process with different ozone-and UV-based AOPs for wastewater treatment at pilot scale: Removal of pharmaceuticals and changes in fluorescing organic matter. Science of The Total Environment. 2021;765:142720.
- Bustillo-Lecompte C, Colina-Marquez J, Rehmann L. Application of Advanced Oxidation Processes. Multidisciplinary Digital Publishing Institute; 2020.
- Elmolla ES, Chaudhuri M, Eltoukhy MM. The use of artificial neural network (ANN) for modeling of COD removal from antibiotic aqueous solution by the Fenton process. Journal of hazardous materials. 2010;179(1-3):127-34.
- Xu Z, Song X, Li Y, Li G, Luo W. Removal of antibiotics by sequencing-batch membrane bioreactor for swine wastewater treatment. Science of The Total Environment. 2019;684:23-30.
- Yang X, Flowers RC, Weinberg HS, Singer PC. Occurrence and removal of pharmaceuticals and personal care products (PPCPs) in an advanced wastewater reclamation plant. Water research. 2011;45(16):5218-28.
- Kovalova L, Siegrist H, Von Gunten U, Eugster J, Hagenbuch M, Wittmer A, et al. Elimination of micropollutants during post-treatment of hospital wastewater with powdered activated carbon, ozone, and UV. Environmental science & technology. 2013;47(14):7899-908.
- Meneau-Hernández RI, Millán-Arrieta JA, Borrego-Morales K, Alba-Carranza MD, Farías-Piñeira T. Adsorción de ciprofloxacina en materiales zeolíticos. Revista Cubana de Química. 2021;33(1):167-90.
- Prieto EdJM, Rivas B, Sánchez J. Natural polymer grafted with syntethic monomer by microwave for water treatment-a review. Ciencia en Desarrollo. 2012;4(1):219-40.
- Homem V, Santos L. Degradation and removal methods of antibiotics from aqueous matrices–a review. Journal of environmental management. 2011;92(10):2304-47.
- Pham TD, Bui TT, Nguyen VT, Bui TKV, Tran TT, Phan QC, et al. Adsorption of polyelectrolyte onto nanosilica synthesized from rice husk: characteristics, mechanisms, and application for antibiotic removal. Polymers. 2018;10(2):220.
- Du J, Zhang B, Li J, Lai B. Decontamination of heavy metal complexes by advanced oxidation processes: A review. Chinese Chemical Letters. 2020;31(10):2575-82.
- Luvita V, Mahardiono NA, Fakhrurroja H, Tanu E, Sugiarto AT. Combination Method of Advanced Oxidation Process (AOPs) and Electromagnetic water treatment (EWT) to reduce heavy metals in PIT Lake. Instrumentasi. 2019;43(2):91-101.
- Vagı M, Petsas A, editors. Advanced oxidation processes for the removal of pesticides from wastewater: recent review and trends. 15th international conference on environmental science and technology CEST2017, Rhodes, Greece; 2017.
- Quiroz MA, Martínez-Huitle CA, Bandala ER. Advanced oxidation processes (AOPs) for removal of pesticides from aqueous media: IntechOpen; 2011.
- Kanakaraju D, Glass BD, Oelgemöller M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. Journal of Environmental Management. 2018;219:189-207.
- Sbardella L, Velo Gala I, Comas J, Morera Carbonell S, Rodríguez-Roda I, Gernjak W. Integrated assessment of sulfate-based AOPs for pharmaceutical active compound removal from wastewater. Journal of Cleaner Production. 2020;260:121014.
- Shukla PR, Wang S, Sun H, Ang HM, Tadé M. Activated carbon supported cobalt catalysts for advanced oxidation of organic contaminants in aqueous solution. Applied Catalysis B: Environmental. 2010;100(3-4):529-34.
- Guo W, Su S, Yi C, Ma Z. Degradation of antibiotics amoxicillin by Co3O4‐catalyzed peroxymonosulfate system. Environmental progress & sustainable energy. 2013;32(2):193-7.
- Kıdak R, Doğan Ş. Medium-high frequency ultrasound and ozone based advanced oxidation for amoxicillin removal in water. Ultrasonics Sonochemistry. 2018;40:131-9.
- Homem V, Alves A, Santos L. Microwave-assisted Fenton’s oxidation of amoxicillin. Chemical Engineering Journal. 2013;220:35-44.
- Elmolla ES, Chaudhuri M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. Journal of hazardous materials. 2010;173(1-3):445-9.
- Klauson D, Babkina J, Stepanova K, Krichevskaya M, Preis S. Aqueous photocatalytic oxidation of amoxicillin. Catalysis Today. 2010;151(1-2):39-45.
- He X, Pelaez M, Westrick JA, O’Shea KE, Hiskia A, Triantis T, et al. Efficient removal of microcystin-LR by UV-C/H2O2 in synthetic and natural water samples. Water research. 2012;46(5):1501-10.
- Dogan S, Kidak R. A plug flow reactor model for UV-based oxidation of amoxicillin. Desalination and Water Treatment. 2016;57(29):13586-99.
- Shahhet L, Alraghban D, Chehna D. Improvement of the physicochemical properties of amoxicillin trihydrate powder by recrystallization at different pH values. Int J Pharm Pharmaceut Sci. 2011;3(suppl 3):92-100.
- Ma J, Gao M, Liu Q, Wang Q. High efficiency three-dimensional electrochemical treatment of amoxicillin wastewater using Mn–Co/GAC particle electrodes and optimization of operating condition. Environmental Research. 2022;209:112728.
- Zhang S, Yang Y-L, Lu J, Zuo X-J, Yang X-L, Song H-L. A review of bioelectrochemical systems for antibiotic removal: Efficient antibiotic removal and dissemination of antibiotic resistance genes. Journal of Water Process Engineering. 2020;37:101421.
- Pirsaheb M, Hossaini H, Raad NK, Kianpour S, Hossini H. A systematic review on photo-Fenton process as an efficient advanced oxidation for degradation of amoxicillin in aqueous environments. Reviews on Environmental Health. 2022.
- Zhang Y, Zhao Y-G, Maqbool F, Hu Y. Removal of antibiotics pollutants in wastewater by UV-based advanced oxidation processes: Influence of water matrix components, processes optimization and application: A review. Journal of Water Process Engineering. 2022;45:102496.
- Arun S, Kothari K, Mazumdar D, Mukhopadhyay M, Chakraborty P. Biochar Production from Domestic Sludge: A Cost-effective, Recycled Product for Removal of Amoxicillin in Wastewater. IOP Conference Series: Materials Science and Engineering. 2017;225:012164.
- Wu H, Feng Q, Yang H, Alam E, Gao B, Gu D. Modified biochar supported Ag/Fe nanoparticles used for removal of cephalexin in solution: Characterization, kinetics and mechanisms. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2017;517:63-71.
- Chakhtouna H, Benzeid H, Zari N, Qaiss Aek, Bouhfid R. Functional CoFe2O4‐modified biochar derived from banana pseudostem as an efficient adsorbent for the removal of amoxicillin from water. Separation and Purification Technology. 2021;266:118592.
- Qi H, Pan G, Shi X, Sun Z. Cu–Fe–FeC3@nitrogen-doped biochar microsphere catalyst derived from CuFe2O4@chitosan for the efficient removal of amoxicillin through the heterogeneous electro-Fenton process. Chemical Engineering Journal. 2022;434:134675.
- Li H, Hu J, Wang X, An L. Development of a bio-inspired photo-recyclable feather carbon adsorbent towards removal of amoxicillin residue in aqueous solutions. Chemical Engineering Journal. 2019;373:1380-8.
- Wang J, Zhuan R. Degradation of antibiotics by advanced oxidation processes: An overview. Science of the Total Environment. 2020;701:135023.
- Cuerda-Correa EM, Alexandre-Franco MF, Fernández-González C. Advanced oxidation processes for the removal of antibiotics from water. An overview. Water. 2019;12(1):102.
- García DE, Delgado N, Aranda FL, Toledo MA, Cabrera-Barjas G, Sintjago EM, et al. Synthesis of maleilated polyflavonoids and lignin as functional bio-based building-blocks. Industrial Crops and Products. 2018;123:154-63.
- Aranda FL, Gayoso A, Palma-Onetto V, Rivas BL. REMOVAL OF COPPER IONS FROM AQUEOUS SOLUTIONS BY USING RESINS FROM PINUS RADIATA BARK RESINS. Journal of the Chilean Chemical Society. 2022;67(1):5403-7.
- Mangla D, Annu, Sharma A, Ikram S. Critical review on adsorptive removal of antibiotics: Present situation, challenges and future perspective. Journal of Hazardous Materials. 2022;425:127946.
- Garcia DE, Glasser WG, Pizzi A, Paczkowski SP, Laborie M-P. Modification of condensed tannins: from polyphenol chemistry to materials engineering. New Journal of Chemistry. 2016;40(1):36-49.
- Schiewer S, Patil SB. Pectin-rich fruit wastes as biosorbents for heavy metal removal: Equilibrium and kinetics. Bioresource Technology. 2008;99(6):1896-903.
- Hosseinzadeh H, Ramin S. Fabrication of starch-graft-poly (acrylamide)/graphene oxide/hydroxyapatite nanocomposite hydrogel adsorbent for removal of malachite green dye from aqueous solution. International journal of biological macromolecules. 2018;106:101-15.
- Tao J, Yang J, Ma C, Li J, Du K, Wei Z, et al. Cellulose nanocrystals/graphene oxide composite for the adsorption and removal of levofloxacin hydrochloride antibiotic from aqueous solution. Royal Society Open Science. 2020;7(10):200857.
- Shamaei L, Karami P, Khorshidi B, Farnood R, Sadrzadeh M. Novel Lignin-Modified Forward Osmosis Membranes: Waste Materials for Wastewater Treatment. ACS Sustainable Chemistry & Engineering. 2021;9(47):15768-79.
- Uddin F. Montmorillonite: An introduction to properties and utilization: IntechOpen London, UK; 2018.
- Wang F, Yang B, Wang H, Song Q, Tan F, Cao Y. Removal of ciprofloxacin from aqueous solution by a magnetic chitosan grafted graphene oxide composite. Journal of Molecular Liquids. 2016;222:188-94.
- Wang T, Pan X, Ben W, Wang J, Hou P, Qiang Z. Adsorptive removal of antibiotics from water using magnetic ion exchange resin. Journal of Environmental Sciences. 2017;52:111-7.
- Rizzi V, Lacalamita D, Gubitosa J, Fini P, Petrella A, Romita R, et al. Removal of tetracycline from polluted water by chitosan-olive pomace adsorbing films. Science of The Total Environment. 2019;693:133620.
- Yan L, Chen W, Wang C, Liu S, Liu C, Yu L, et al. Tetracycline removal in granulation: Influence of extracellular polymers substances, structure, and metabolic function of microbial community. Chemosphere. 2022;288:132510.
- Olasupo A, Sadiq AC, Suah FBM. A novel approach in the removal of ciprofloxacin antibiotic in an aquatic system using polymer inclusion membrane. Environmental Technology & Innovation. 2022;27:102523.
- Qiu L, Jaria G, Gil MV, Feng J, Dai Y, Esteves VI, et al. Core− shell molecularly imprinted polymers on magnetic yeast for the removal of sulfamethoxazole from water. Polymers. 2020;12(6):1385.
- Neghi N, Kumar M, Burkhalov D. Synthesis and application of stable, reusable TiO2 polymeric composites for photocatalytic removal of metronidazole: Removal kinetics and density functional analysis. Chemical Engineering Journal. 2019;359:963-75.
- Dai J, He J, Xie A, Gao L, Pan J, Chen X, et al. Novel pitaya-inspired well-defined core–shell nanospheres with ultrathin surface imprinted nanofilm from magnetic mesoporous nanosilica for highly efficient chloramphenicol removal. Chemical Engineering Journal. 2016;284:812-22.
- Kuru CI, Ulucan F, Kuşat K, Akgöl S. A model study by using polymeric molecular imprinting nanomaterials for removal of penicillin G. Environmental Monitoring and Assessment. 2020;192(6):1-16.
- Bajpai S, Jhariya S. Selective removal of Amikacin from simulated polluted water using molecularly imprinting polymer (MIP). Journal of Macromolecular Science, Part A. 2015;52(11):901-11.
- Prasannamedha G, Kumar PS, Shivaani S, Kokila M. Sodium alginate/magnetic hydrogel microspheres from sugarcane bagasse for removal of sulfamethoxazole from sewage water: Batch and column modeling. Environmental Pollution. 2022;307:119523.
- Rivas-Sanchez A, Cruz-Cruz A, Gallareta-Olivares G, González-González RB, Parra-Saldívar R, Iqbal HM. Carbon-based nanocomposite materials with multifunctional attributes for environmental remediation of emerging pollutants. Chemosphere. 2022:135054.
- Zhang X, Lin X, Ding H, He Y, Yang H, Chen Y, et al. Novel alginate particles decorated with nickel for enhancing ciprofloxacin removal: Characterization and mechanism analysis. Ecotoxicology and Environmental Safety. 2019;169:392-401.
- Karimi S, Namazi H. Magnetic alginate/glycodendrimer beads for efficient removal of tetracycline and amoxicillin from aqueous solutions. International Journal of Biological Macromolecules. 2022;205:128-40.
- Kaur A, Maity C. Amoxicillin removal from an aqueous solution by adsorption using graphene oxide/calcium alginate biocomposite. Journal of Physics: Conference Series. 2020;1531(1):012109.
- H. Ragab A, Hussein HS, Ahmed IA, Abualnaja KM, AlMasoud N. An Efficient Strategy for Enhancing the Adsorption of Antibiotics and Drugs from Aqueous Solutions Using an Effective Limestone-Activated Carbon–Alginate Nanocomposite. Molecules. 2021;26(17):5180.
- Rusu L, Grigoraș C-G, Simion A-I, Suceveanu EM, Șuteu D, Harja M. Application of Saccharomyces cerevisiae/Calcium Alginate Composite Beads for Cephalexin Antibiotic Biosorption from Aqueous Solutions. Materials. 2021;14(16):4728.
- Shakil MS, Mahmud KM, Sayem M, Niloy MS, Halder SK, Hossen M, et al. Using Chitosan or Chitosan Derivatives in Cancer Therapy. Polysaccharides. 2021;2(4):795-816.
- Mincea M, Negrulescu A, Ostafe V. Preparation, modification, and applications of chitin nanowhiskers: a review. Rev Adv Mater Sci. 2012;30(3):225-42.
- Ardean C, Davidescu CM, Nemeş NS, Negrea A, Ciopec M, Duteanu N, et al. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. International Journal of Molecular Sciences. 2021;22(14):7449.
- Sashiwa H, Aiba S-i. Chemically modified chitin and chitosan as biomaterials. Progress in polymer science. 2004;29(9):887-908.
- Sivanesan I, Gopal J, Muthu M, Shin J, Mari S, Oh J. Green Synthesized Chitosan/Chitosan Nanoforms/Nanocomposites for Drug Delivery Applications. Polymers. 2021;13(14):2256.
- Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Advanced Drug Delivery Reviews. 2001;51(1):81-96.
- Iqbal DN, Shafiq S, Khan SM, Ibrahim SM, Abubshait SA, Nazir A, et al. Novel chitosan/guar gum/PVA hydrogel: Preparation, characterization and antimicrobial activity evaluation. International Journal of Biological Macromolecules. 2020;164:499-509.
- Vikash R, Mitthra S, Tamilselvi R, Vivekanandhan P. Clinical Applications Of Chitosan In Dentistry-A Review Chitosan In Dentistry.
- Jiang X, Xiang N, Wang J, Zhao Y, Hou L. Preparation and characterization of hybrid double network chitosan/poly (acrylic amide-acrylic acid) high toughness hydrogel through Al3+ crosslinking. Carbohydrate polymers. 2017;173:701-6.
- Danalıoğlu ST, Bayazit ŞS, Kuyumcu ÖK, Salam MA. Efficient removal of antibiotics by a novel magnetic adsorbent: Magnetic activated carbon/chitosan (MACC) nanocomposite. Journal of Molecular Liquids. 2017;240:589-96.
- Noel SP, Courtney H, Bumgardner JD, Haggard WO. Chitosan films: a potential local drug delivery system for antibiotics. Clinical orthopaedics and related research. 2008;466(6):1377-82.
- Yang Z, Hou T, Ma J, Yuan B, Tian Z, Yang W, et al. Role of moderately hydrophobic chitosan flocculants in the removal of trace antibiotics from water and membrane fouling control. Water research. 2020;177:115775.
- Karimi-Maleh H, Ayati A, Davoodi R, Tanhaei B, Karimi F, Malekmohammadi S, et al. Recent advances in using of chitosan-based adsorbents for removal of pharmaceutical contaminants: A review. Journal of Cleaner Production. 2021:125880.
- Palacio DA, Urbano BF, Rivas BL. Hydrogels based on alkylated chitosan and polyelectrolyte copolymers. Journal of Applied Polymer Science. 2018;135(31):46556.
- Ju S, Shin G, Lee M, Koo JM, Jeon H, Ok YS, et al. Biodegradable chito-beads replacing non-biodegradable microplastics for cosmetics. Green Chemistry. 2021;23(18):6953-65.
- Amen R, Bashir H, Bibi I, Shaheen SM, Niazi NK, Shahid M, et al. A critical review on arsenic removal from water using biochar-based sorbents: the significance of modification and redox reactions. Chemical Engineering Journal. 2020;396:125195.
- Dutta J, Mala AA. Removal of antibiotic from the water environment by the adsorption technologies: a review. Water Science and Technology. 2020;82(3):401-26.
- Yaqubi O, Tai MH, Mitra D, Gerente C, Neoh KG, Wang C-H, et al. Adsorptive removal of tetracycline and amoxicillin from aqueous solution by leached carbon black waste and chitosan-carbon composite beads. Journal of Environmental Chemical Engineering. 2021;9(1):104988.
- Heydaripour J, Gazi M, Oladipo AA, Gulcan HO. Porous magnetic resin-g-chitosan beads for adsorptive removal of phenolic compounds. International journal of biological macromolecules. 2019;123:1125-31.
- Philippova O, Korchagina E. Chitosan and its hydrophobic derivatives: Preparation and aggregation in dilute aqueous solutions. Polymer Science Series A. 2012;54(7):552-72.
- Roy JC, Salaün F, Giraud S, Ferri A, Chen G, Guan J. Solubility of chitin: solvents, solution behaviors and their related mechanisms. Solubility of polysaccharides. 2017;3:20-60.
- Wadhwa S, Paliwal R, Rai Paliwal S, P Vyas S. Chitosan and its role in ocular therapeutics. Mini reviews in medicinal chemistry. 2009;9(14):1639-47.
- Danalıoğlu ST, Bayazit ŞS, Kerkez Kuyumcu Ö, Salam MA. Efficient removal of antibiotics by a novel magnetic adsorbent: Magnetic activated carbon/chitosan (MACC) nanocomposite. Journal of Molecular Liquids. 2017;240:589-96.
- Weng X, Lin S, Zhong Y, Chen Z. Chitosan stabilized bimetallic Fe/Ni nanoparticles used to remove mixed contaminants-amoxicillin and Cd (II) from aqueous solutions. Chemical Engineering Journal. 2013;229:27-34.
- Gupta V, Nayak A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chemical engineering journal. 2012;180:81-90.
- Shi L-n, Zhang X, Chen Z-l. Removal of chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water research. 2011;45(2):886-92.
- Yuwei C, Jianlong W. Preparation and characterization of magnetic chitosan nanoparticles and its application for Cu (II) removal. Chemical engineering journal. 2011;168(1):286-92.

