PHYTOCHEMICAL STUDY ON ECTOMYCORRHIZAL FUNGI Cortinarius magellanicus: AN UNUSUAL BROMINATED SECONDARY METABOLITE ISOLATED

- Brominated saccharide,
- Cortinarius magellanicus,
- metabolite characterization,
- Nothofagus ectomycorrhizal fungi,
- phytochemical analysis
Copyright (c) 2023 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
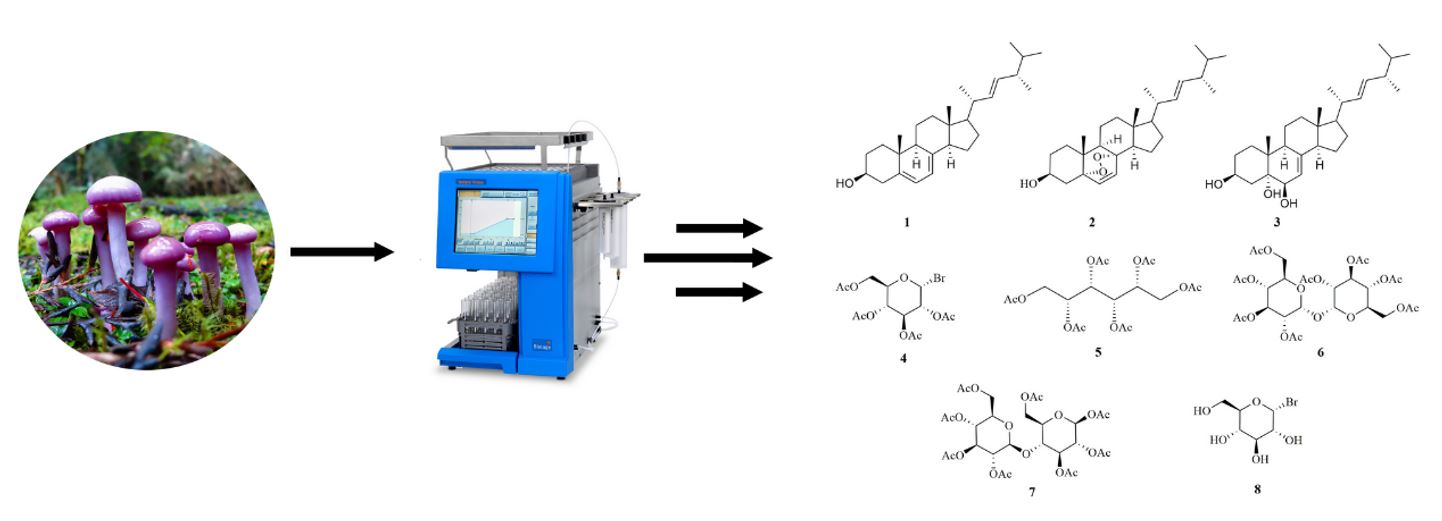
Phytochemical analysis of the basidiomycete Cortinarius magellanicus (family Cortinariaceae) resulted in the isolation of eight secondary metabolites, where a brominated secondary metabolite was isolated for first time from a natural source. The structure elucidation of this compound was made using one- and two-dimensional NMR experiments, FT-IR, GC-MS, HRESIMS and chemical derivation. The disk diffusion assay of the brominated compound 4 and 8 revealed a low inhibition on Gram-positive and Gram-negative bacteria, respectively. No antifungal activities were detected for these compounds.
References
- Beattie KD, Rouf R, Gander L, May TW, Ratkowsky D, Donner CD, Gill M, Grice ID, Tiralongo E. Antibacterial metabolites from Australian macrofungi from the genus Cortinarius. Phytochemistry. 71(8–9):948–955, (2010).
- Beattie KD, Thompson DR, Tiralongo E, Ratkowsky D, May TW, Gill M. Austrocolorone B and austrocolorin B1, cytotoxic anthracenone dimers from the Tasmanian mushroom Cortinarius vinosipes Gasparini. Tetrahedron Lett. 52(42):5448–5451, (2011).
- Beekman AM, Wossa SW, Kevo O, Ma P, Barrow RA. Discovery and Synthesis of Boletopsins 13 and 14, Brominated Fungal Metabolites of Terrestrial Origin. J Nat Prod. 78(8):2133–2135. (2015).
- Deacon J. Fungal Biology. Blackwell. Publishing B, editor. Blackwell publishing: Blackwell publishing. 2006.
- Dembitsky V, Tolstikov G. Natural Halogenated Mononuclear Phenol Compounds and Their Derivatives. Chem Sustain Dev. 11:567–575, (2003).
- Fehér D, Barlow R, McAtee J, Hemscheidt TK. Highly Brominated Antimicrobial Metabolites from a Marine Pseudoalteromonas sp . J Nat Prod. 73(11):1963–1966, (2010).
- Geissler T, Brandt W, Porzel A, Schlenzig D, Kehlen A, Wessjohann L, Arnold N. Acetylcholinesterase inhibitors from the toadstool Cortinarius infractus. Bioorganic Med Chem. 18(6):2173–2177, (2010).
- Gottardi W, Klotz S, Nagl M. Superior bactericidal activity of N-bromine compounds compared to their N-chlorine analogues can be reversed under protein load. J Appl Microbiol. 116(6):1427–1437, (2014).
- Gurst JE. NMR and the Structure of D-Glucose. Textb Forum. 68(12):1003–1004, (1991).
- Heleno S a., Barros L, Sousa MJ, Martins A, Ferreira ICFR. Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem. 119(4):1443–1450, (2010).
- Igarashi K. The Koenigs-Knorr Reaction. p. 243–283, (1977).
- Kehoe MA. Chapter 11 Cell-wall-associated proteins in Gram-positive bacteria. New Compr Biochem. 27(C):217–261, (1994).
- Koronakis V, Hughes C. Chapter 20 Secretion of hemolysin and other proteins out of the Gram-negative bacterial cell. New Compr Biochem. 27(C):425–446, (1994).
- Lin Chan Ching, Huang LC, Liang PH, Liu CY, Lin Chun Cheng. Large-scale synthesis of per-O-acetylated saccharides and their sequential transformation to glycosyl bromides and thioglycosides. J Carbohydr Chem. 25(4):303–313, (2006).
- Matin MM, Bhuiyan MMH, Afrin A, Debnath DC. Comparative Antimicrobial Activities of some Monosaccharide and Disaccharide Acetates. J Sci Res. 5(3):515–525, (2013).
- Ministerio del medio ambiente Chile. Ficha de antecedentes Cortinarius magellanicus Spegazzini. :1–5. 2014.
- Nicholas GM, Blunt JW, Munro MHG. Cortamidine oxide, a novel disulfide metabolite from the New Zealand basidiomycete (mushroom) Cortinarius species. J Nat Prod. 64:341–344, (2001).
- Niveiro N, Albertó E. Checklist of the Argentine Agaricales 7. Cortinariaceae and Entolomataceae. Check List. 10(1):72, (2014).
- Odlaug TE. Antimicrobial Activity of Halogens. J Food Prot. 44(8):608–613, (1981).
- Poinsot V, Carpene MA, Couderc F. Coupled Mass Spectrometric Strategies for the Determination of Carbohydrates at Very Low Concentrations: The Case of Polysaccharides Involved in the Molecular Dialogue Between Plants and Rhizobia. The Complex World of Polysaccharides. 2012.
- Pomin VH. Unravelling Glycobiology by NMR Spectroscopy. Intech.:63–98. (2012).
- Sande D, de Oliveira GP, Moura MAF., Martins B de A, Lima MTNS, Takahashi JA. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res Int. 125(March):108524, (2019).
- Semon E, Mauvais G, De Jong E, Spinnler H, Le Quere J. Production of halogenated compounds by Bjerkandera adusta. Appl Microbiol Biotechnol. 42:212–221, (1994).
- Sontag B, Frode R, Bross M, Steglich W. Chromogenic triterpenoids from Cortinarius fulvoincarnatus, C-sodagnitus and related toadstools (Agaricales). European J Org Chem.:255–260, (1999).
- Tan JW, Dong ZJ, Liu JK. New cerebrosides from the basidiomycete Cortinarius tenuipes. Lipids. 38(1):81–84, (2003).