- Chalcones,
- Cyclic Voltammetry,
- DFT calculations,
- HOMO-LUMO levels,
- optoelectronic materials
Copyright (c) 2022 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
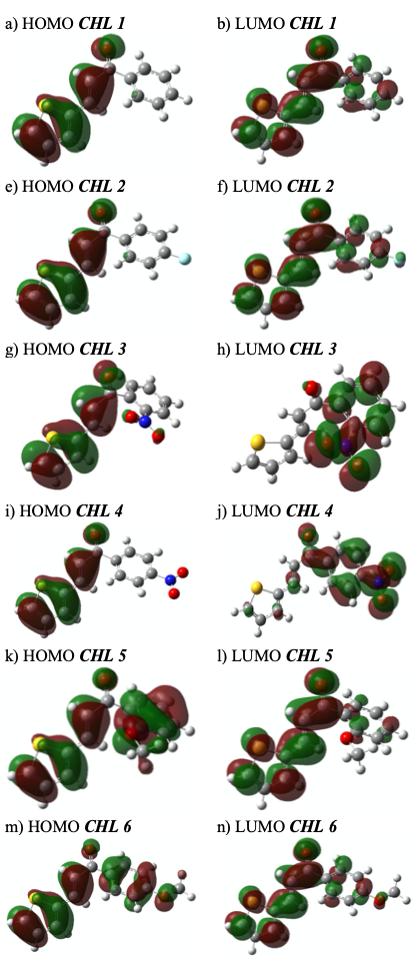
We have studied the electronic and electrochemical properties of seven substituted heterocyclic chalcones (CHL 1-6) wich are functionalized with 2-thienyl groups. The phenyl ring of the chalcone scaffold has been substituted in orto or para position with electron donnor and acceptor groups (-F, -NO2, -CH3O). The electrochemical features were analyzed by cyclic voltammetry; irreversible processes were observed and attributed to the oxidation and reduction of the 2-thienyl group and the carbonyl group of the chalcone, respectively. DFT calculations were made to gain more in-depth understanding of their electronic properties. Relatively high HOMO level and low LUMO values were calculated, Eg values computed varies between 3.0-3.29 eV. These values enable us to postulate that these type of compounds are suitable building blocks to fabricate optoelectronic devices.
References
[1] B.G.M. Youssif, International Journal of Pharmaceutical Sciences and Research, 10 (5) (2019) 2423-2429.
[2] E.M. Meshram, B.N. Berad, CH. P. Pandhurnekar, H.N. Chopde, International Journal of Pharma and Bio Sciences, 9 (4) (2018) 189-194.
[3] G. Vanangamudi; R. Sundararajan; R. Arulkumaran; S. Vijayakumar; G. Thirunarayanan, Der Pharma Chemica, 5 (6) (2013) 213-224.
[4] O.A. Nurkenov; M.K. Ibraev; I. A. Schepetkin; A.I. Khlebnikov; T.M. Seilkhanov; A.E. Arinova; M.B. Isabaeva, Russian Journal of General Chemistry 89(7) (2019) 1360-1367.
[5] M.S. Salem; R.A. Hussein; W. M. El-Sayed, Anti-Cancer Agents in Medicinal Chemistry 19(5) (2019) 620-626.
[6] G.D.C. d'Oliveira; J.M.F. Custodio; A.F. Moura; H.B. Napolitano; C.N. Perez; M.O Moraes; L. Prokai; P. Perjesi, Medicinal Chemistry Research, 28(9) (2019) 1448-1460.
[7] M. Khanusiya; Z. Gadhawala, Journal of the Korean Chemical Society, 63(2) (2019) 85-93.
[8] P.S. Patil; S.R. Maidur; J.R. Jahagirdar, T. S. Chia; Ch. K. Quah; M. Shkir, Applied Physics B: Lasers and Optics, 125(9) (2019) 1-13.
[9] S.R Maidur; P. S. Patil; Optik, 190 (2019) 54-67.
[10] A. Ekbote, Anusha; P.S. Patil; S.R. Maidur; T. S. Chia; C. K. Quah, Journal of Molecular Structure, 1129 (2017) 239-247.
[11] A. N. Prabhu, V. Upadhyaya, A. Jayarama, K. Subrahmanya Bhat, Molecular Crystals and Liquid Crystals, 637 (1) (2016) 76-86 (2016).
[12] A.I. Daud; W.M. Khairul; E. Augustine; S. Arshad; I. A. Razak, Ibrahim, Journal of Molecular Structure, 1194 (2019) 124-137.
[13] A. Karuppusamy, T. Vandana, P. Kannan, Journal of Photochemistry and Photobiology A: Chemistry, 345 (2017) 11-20.
[14] A. R. Chaudhry, A. Irfan, S. Muhammad, A. G. Al-Sehemi, Journal of Molecular Graphics and Modelling, 75 (2017) 355-364.
[15] P. Ganesan, D.G. Chen, J.L. Liao, W.Ch. Li, Y. N. Lai, D. Luo, Ch.H. Chang, Ch. L. Ko, W.Y. Hung, Sh. W. Liu, G. H. Lee, P. T Chou, Y. Chi, J. Mater.Chem., C., 6 (2018) 10088-10100.
[16] Q. Zhang, Sh. Sun, X. Lv, W. Liu, H. Zeng, R. Guo, Sh. Ye, P. Leng, S. Xiang, L. Wang, Mater.Chem.Front, 2(11) (2018) 2054-2062.
[17] S. Surech, N. Bhuvanesh, J. Prabhu, A. Thamilselvan, S. Rex Jeya Rajkumar, K. Kannan, V. Rajesh Kannan, R. Nandhakumar, Journal of Photochemistry and Photobiology A: Chemistry 359 (2018) 172-182.
[18] H. Karaca, B. Çayegil, S. Sezer, Synthetic Metals, 215 (2016) 134-141.
[19] A Kamal, K. Kumar, V Kumar, R. Kumar Mahajan, Electrchimica Acta, 145 (2014) 307-313.
[20] B. Delavaux-Nicot, J.Maynadié, D. Lavabre, S. Fery-Forgues, J. Organomet. Chem. 692 (4) (2007) 874-886.
[21] Tushar M. Khopade, Prakash K. Warghude, Trimbak B. Mete and Ramakrishna G. Bhat, Tetrahedron Letters 60(2) (2019) 197-200.
[22] Chang-Ji Zheng, Sheng-Ming Jiang, Zhen-Hua Chen, Bai-Jun Ye, and Hu-Ri Piao, Arch. Pharm. Chem. Life Sci. 344(10) (2011) 689-695.
[23] Ebru Mete, Birnur Comez, Halise Inci Gul, Ilhami Gulcin, Claudiu T. Supuran, J Enzyme Inhib Med Chem. 31(Sup 2) (2016) 1-5.
[24] V. Colotta, D. Catarzi, F. Varano, G. Filacchioni, L Cecchi, A. Galli, Ch. Costagli J. Med. Chem. 39(15) (1996), 2915-2921.
[25], F. Brovelli, R. Baggio, L. Alvarez, Y. Moreno, Journal of Chilean Chemical Society 66 (2) (2021) 5190-5194.
[26] I. F. Hu, D. H. Karweik, T. Kuwana, J. Electroanal. Chem., 188(1) (1985) 59-72.
[27] G. W. Hance, T. Kuwana, Anal. Chem, 59 (1) (1987) 131-134.
[28] Gaussian 09, Revision A.01, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian, Inc., Wallingford CT, (2009).
[29] J. P. Perdew, Phys. Rev. B 33(12) (1986) 8822-8824.
[30] J. P. Perdew, Phys. Rev. B 34(10) (1986) 7406-7406.
[31] W. R. Wadt and P. J. Hay, J. Chem. Phys. 82 (1985) 270-283.
[32] A.V. Marenich, C.J. Cramer, D.G. Truhlar. J. Phys. Chem. B 113(18) (2009) 6378-6396.
[33] R. Baggio, F. Brovelli, Y. Moreno, M. Pinto, J. Soto-Delgado, Journal of Molecular Structure 1123 (2016) 1-7.
[34] M. Dhananjayulu, M. Siva Prasad, Ch. Swarupa, M. Seenu Naik, N.Y. Sreedhar, International Journal of Innovative Research in Science, Engineering and Technology, 3 (5) (2014) 12496-12501.
[35] S. Wawzonek, A. Gundersen, J. Electrochem. Soc. 3(3) (1964) 324-328
[36] J.P Zimmer, J. A. Richards, J. C. Turner, D. H. Evans, Anal. Chem, 43(8) (1970) 1000-1006.
[37] R. S. Nicholson, I. Shain, Anal. Chem. 36(4) (1964) 706-726
[38] P. Zuman, Substituent Effects in Organic Polarography, Plenum Press, New York, (1967).
[39] R. A. Ford, A. J. Gordon The Chemist's Companion: A Handbook of Practical Data, Techniques, and References, Wiley Editions (1973).
[40] J. Y. Alston, A. J. Fry, Electrochimica Acta 49 (2004) 455-459
[41] Ebenezar, J. D., Ramalingam, S., Raja, C. R. & Helan, V. J. Theo. Comput. Sci. 1(2) (2013) 1-13.
[42] B. E. Aksöz, R. Ertan, FABAD J. Pharm. Sci. 37 (4) (2012) 205-216.
[43] M. Oumi, D. Maurice, M. Head-Gordon, Spectrochimica Acta Part A, 55(3) (1999) 525-537.
[44] R. Schlaf, P. G. Schroeder, M. W. Nelson, B. A. Parkinson, C. D. Merritt, L. L. A. Crisafulli, H. Murata, Z. H. Kafafi, Surface Science, 450(1-2) (2000)142-152.
[45] P.I. Djurovich, E.I.Mayo, S.R. Forrest, M.E. Thompson, Organic Electronics 10(3) (2009) 515–520 .
[46] M. Mohamed Ahmida, S. H. Eichborn, ECS Transactions, 25(26) (2010) 1-10.
[47] B.W. D’Andrade, S. Datta, S.R. Forrest, P. Djurovich, E. Polikarpov, M.E. Thompson Org. Electron. 6 (2005)11-20.
[48] C.M. Cardona, W. Li, A.E. Kaifer, D. Stockdale, G.C. Bazan Adv. Mater. 23(20) (2011) 2367-2371.
[49] J.L. Bredas, R. Silbey, D.S. Boudreux, R.R. Chance, J. Am. Chem. Soc. 105(22) (1983) 6555-6559.
[50] T. J. Cleij, J. K. King, L. W. Jenneskens, Chem. Mater. 12(1) (2000) 84-89.
[51] T. J. Cleij, J. K. King and L. W. Jenneskens, Macromolecules 33(1) (2000) 89-96.
[52] L. Leonat, G. Sbârcea, I. Viorel Brânzoi, U.P.B.Sci. Bull., Series B, 75 (3) (2013) 111-118.
[53] K. Tremel, S. Ludwigs Adv. Polym. Sci., 265 (2014) 39-82
[54] P. Acevedo-Peña, A. Baray-Calderón, H. Hu, I. González, V.M. Ugalde-Saldivar, J. Solid State Electrochem., 21 (2017) 2407-2414
[55] P.J. Tejkiran, M.S. Brahma Teja, P Sai Siva Kumar, P. Sankar, R. Philip, S. Naveen, N.K. Lokanath, G. Nageswara Rao J. Photochemistry Photobiology A: Chemistry 324 (2016) 33-39.
[56] S. Sathish, B. Chandar Shekar, S. Chandru Kannan, R. Sengodan, K.P.B. Dinesh, R. Ranjithkumar Int. J. Polym. Anal. Charact. 20(1) (2015) 29-41.
[57] Dian Alwani Zainuri, Ibrahim Abdul Razak and Suhana Arshad, Acta Cryst. E Crystallogr. Comun. 74 (10) (2018) 1491-1496.
[58] Ainizatul Husna Anizaim, Muhamad Fikri Zaini, Muhammad Adlan Laruna, Ibrahim Abdul Razak, S. Arshad Acta Cryst. E Crystallogr. Comun. 75(5) (2019) 632-637.