- Sophora,
- secondary metabolites,
- alkaloids,
- flavonoids,
- chalcones
Copyright (c) 2022 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
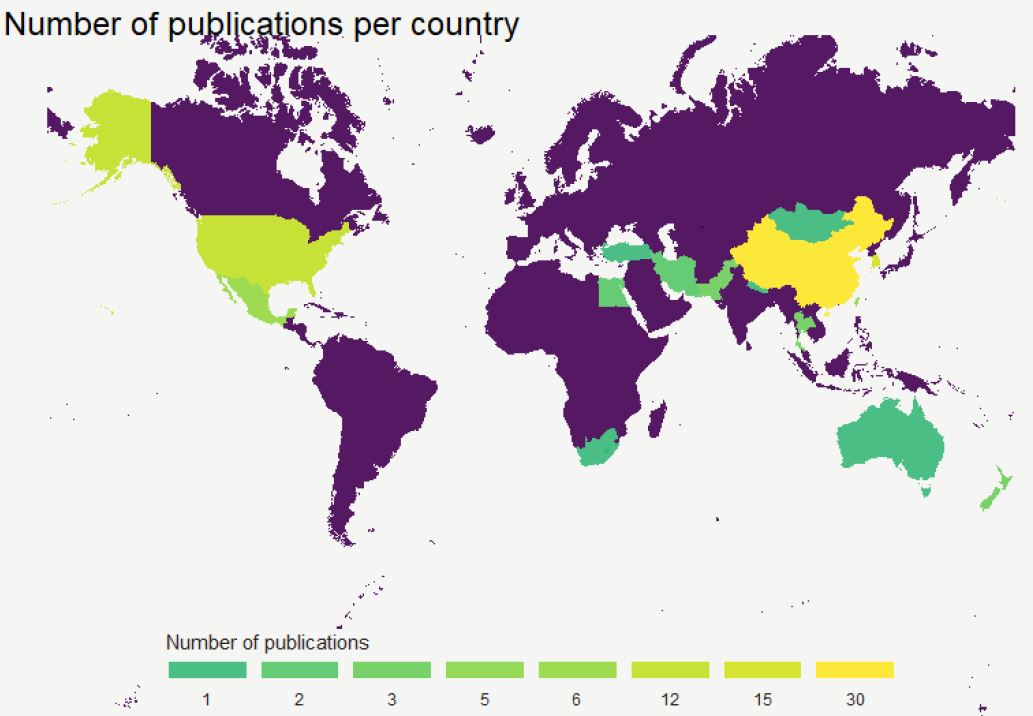
The genus Sophora (Fabaceae) has been used by different cultures for medicinal and ceremonial reasons. This has led to describe and isolate the compounds present in the different parts of different species. In this review, we surveyed the secondary metabolites present in 20 different species of Sophora, which have been identified, isolated, and purified between 1969 and 2020. The aims of this review are demonstrate the spatial temporal evolution of research in the identification of secondary metabolites in the genus Sophora and determine the secondary metabolites identified and classified in the different species of the genus Sophora which may serve as a potential guide for future research. The countries with the highest number of species studied are China, South Korea, and the United States of America. The species that presented a greater identification of compounds are S. alopecuroides, S. secundiflora, S. tonkinensis and S. flaveceses, presenting a wide variety of compounds such as alkaloids, flavonoids, flavones, flavanones, chalcones, among others. The most studied plant parts in research are the roots, seed, and leaves, but in some species only one part was studied, leaving the possibility of studying the rest of the plant. And the difference in the presence of compounds in species that are present in different geographic positions should be considered for future studies.
References
- U.Quattrocchi. CRC World Dictionary of Medicinal and Poisonous Plants. Common Names, Scientific Names, Eponysm, Synonyms, and Eymology, Taylor & Francis Group, Boca Raton, 2012.
- M. Boozari, S. Soltani, M. Iranshahi, Phyther Res. 33, 546, (2019).
- J. L. Diaz, Annu. Rev. Pharmacol. Toxicol. 17, 647 (1977).
- Q. Abbas, S. W. Khan, S. Khatoon, S. A. Hussain, S. N. ul Hassan, A. Hussain, R. Qureshi, J. Biodivers. Environ. Sci. 5, 75, (2014).
- S. Farooq, A. Barki, M. Y. Khan, H. Fazal, Pakistan J. Weed Sci. Res. 18, 277, (2012).
- A. B. Gulshan, A. A. Dasti, S. Hussain, M. I. Atta, M. Amin, ARPN J. Agric. Biol. Sci. 7, 750, (2012).
- I. Hussain, A. Bano, F. Ullah, Pakistan J. Bot. 43, 79, (2011).
- N. Jabeen, M. Ajaib, M. F. Siddiqui, M. Ulfat, B. Khan, FUUAST J. Biol. 5, 153, (2015).
- R. Kunwar, C. Burlakoti, C. Chowdhary, R. Bussmann, Med Aromat Plant Sci Biotechnol 4, 28, (2010).
- C.X. Wan, J.G. Luo, X.P. Ren, L.-Y. Kong, Phytochemistry 116, 290, (2015).
- J. Kwon, S. Basnet, J. W. Lee, E.-K. Seo, N. Tsevegsuren, B. Y. Hwang, D. Lee, Bioorg. Med. Chem. Lett. 25, 3314, (2015).
- K. Khan, J. Alam, H. Ali, H. Ahmad, S. Muhammad, Spec. J. Biol. Sci. 3, 16, (2017).
- G. M. Hatfield, L. J. Valdes, W. J. Keller, W. L. Merrill, V. H. Jones, Lloydia. 40, 374, (1977).
- A. Chang, Z. Cai, Z. Wang, S. Sun, African J. Tradit. Complement. Altern. Med. 11, 245, (2014).
- Atta-ur-Rahman, M. I. Choudhary, K. Parvez, A. Ahmed, F. Akhtar, M. Nur-e-Alam, N. M. Hassan, J. Nat. Prod. 63, 190, (2000).
- B. Cai, N. Chen, J. Li, Z. Fang, G. Wang, X. Wang, X. Tan, T. Ban, Y. Lu, J. Med. Plants Res. 5, 2682, (2011).
- Z. Dang, L. Zhu, W. Lai, H. Bogerd, K.-H. Lee, L. Huang, C.-H. Chen, ACS Med. Chem. Lett. 7, 240, (2016).
- N. Goodarzi, E. Doorgard, P. Pournaghi, Res. J. Pharmacogn. 5, 47, (2017).
- C. Guo, L. Yang, C.-X. Wan, Y.-Z. Xia, C. Zhang, M.-H. Chen, Z.-D. Wang, Z.-R. Li, X.-M. Li, Y.-D. Geng, L.-Y. Kong, Phytomedicine. 23, 1629, (2016).
- C. Guo, L. Yang, J. Luo, C. Zhang, Y. Xia, T. Ma, L. Kong, Int. Immunopharmacol. 38, 349, (2016).
- M. Iinuma, M. Ohyama, T. Tanaka, Phytochemistry. 38, 519, (1995).
- Y.-F. Jiao, M. Lu, Y.-P. Zhao, N. Liu, Y.-T. Niu, Y. Niu, R. Zhou, J.-Q. Yu, Molecules. 23, 510, (2018).
- S. Kianbakht, R. Hajiaghaee, S. Akhondzadeh, Phyther. Res. 34, 1108, (2020).
- Z. J. Rong, G. S. Hu, S. Y. Lin, T. Yan, N. Li, Y. Zhao, J. M. Jia, A. H. Wang, Molecules. 25, 2, (2020).
- T. Tanaka, M. Ohyama, M. Iinuma, Y. Shirataki, M. Komatsu, B. Charles L., Phytochemistry. 48, 1187, (1998).
- Y. Huang, J. Hao, D. Tian, Y. Wen, P. Zhao, H. Chen, Y. Lv, X. Yang, Front. Pharmacol. 9, 1, (2018).
- M. Ohyama, M. Ichise, T. Tanaka, M. Iinuma, C. L. Burandt, Tetrahedron Lett., 37, 5155, (1996).
- S. Takamatsu, K. Saito, S. Ohmiya, N. Ruangrungsi, I. Murakoshi, Phytochemistry. 30, 3793, (1991).
- M. Iinuma, J. Yokoyama, M. Ohyama, T. Tanaka, M. Mizuno, N. Ruangrungsi, Phytochemistry. 33, 203, (1993).
- N. Ruangrungsi, M. Iinuma, T. Tanaka, M. Ohyama, J. Yokoyama, M. Mizuno, Phytochemistry. 31, 999, (1992).
- Y. Cai, Q. Luo, M. Sun, H. Corke, Life Sci. 74, 2157, (2004).
- S.-M. Cha, J.-D. Cha, E.-J. Jang, G.-U. Kim, K.-Y. Lee, Arch. Oral Biol. 68, 97, (2016).
- P.-L. Ding, Z.-X. Liao, H. Huang, P. Zhou, D.-F. Chen, Bioorg. Med. Chem. Lett. 16, 1231, (2006).
- X.-H. Ge, L. Shao, G.-J. Zhu, Metab. Brain Dis. 33, 1869, (2018).
- Q. Huang, L. Xu, W.-S. Qu, Z.-H. Ye, W.-Y. Huang, L.-Y. Liu, J.-F. Lin, S. Li, H.-Y. Ma, Eur. Food Res. Technol. 243, 1127, (2017).
- W.-C. Huang, P.-Y. Gu, L.-W. Fang, Y.-L. Huang, C.-F. Lin, C.-J. Liou, Phytomedicine. 61, 152852, (2019).
- E. M. Hwang, Y. B. Ryu, H. Y. Kim, D.-G. Kim, S.-G. Hong, J. H. Lee, M. J. Curtis-Long, S. H. Jeong, J.-Y. Park, K. H. Park, Bioorg. Med. Chem. 16, 6669, (2008).
- H. A. Jung, S. E. Jin, R. J. Choi, H. T. Manh, Y. S. Kim, B.-S. Min, Y. K. Son, B. R. Ahn, B.-W. Kim, H. S. Sohn, J. S. Choi, Arch. Pharm. Res. 34, 2087, (2011).
- H. A. Jung, S. E. Jin, J.-S. Park, J. S. Choi, Phyther. Res. 25, 709, (2011).
- S. S. Kang, J. S. Kim, K. H. Son, H. W. Chang, H. P. Kim, Fitoterapia. 71, 511, (2000).
- C. W. Kang, N. H. Kim, H. A. Jung, H. W. Choi, M. J. Kang, J. S. Choi, G. Do Kim, Environ. Toxicol. Pharmacol. 43, 140, (2016).
- B. H. Kim, K. M. Na, I. Oh, I. H. Song, Y. S. Lee, J. Shin, T. Y. Kim, Biochem. Pharmacol. 85, 1134, (2013).
- C. S. Kim, S.-N. Park, S.-J. Ahn, Y.-W. Seo, Y.-J. Lee, Y. K. Lim, M. O. Freire, E. Cho, J.-K. Kook, Anaerobe. 19, 17, (2013).
- C. Y. Kim, H. J. Kim, K.-M. Kim, M.-H. Oak, Biosci. Biotechnol. Biochem. 77, 395, (2013).
- J. H. Kim, I. S. Cho, Y. K. So, H.-H. Kim, Y. H. Kim, J. Enzyme Inhib. Med. Chem. 33, 1048, (2018).
- L.-P. Pu, H.-P. Chen, M.-A. Cao, X.-L. Zhang, Q.-X. Gao, C.-S. Yuan, C.-M. Wang, Life Sci. 93, 791, (2013).
- T. H. Quang, N. T. T. Ngan, C. Van Minh, P. Van Kiem, B. H. Tai, N. X. Nhiem, N. P. Thao, B. T. T. Luyen, S. Y. Yang, Y. H. Kim, Phyther. Res. 27, 1300, (2013).
- Z.-Y. Wun, C.-F. Lin, W.-C. Huang, Y.-L. Huang, P.-Y. Xu, W.-T. Chang, S.-J. Wu, C.-J. Liou, Food Chem. Toxicol. 62, 255, (2013).
- S.-Y. Zhang, W. Li, H. Nie, M. Liao, B. Qiu, Y.-L. Yang, Y.-F. Chen, Chem. Biodivers. 15, 1, (2018).
- M. Iinuma, M. Ohyama, T. Tanaka, M. P. Hegarty, E. E. Egarty, Phytochemistry. 34, 1654, (1993).
- H. M. Abdallah, A. M. Al-Abd, G. F. Asaad, A. B. Abdel-Naim, A. M. El-halawany, PLoS One. 9, 1, (2014).
- M. I. S. Abdelhady, A. M. Kamal, S. M. Othman, M. S. Mubarak, T. Ben Hadda, Med. Chem. Res. 24, 482, (2015).
- X.-Q. Dong, Q. Du, W.-H. Yu, Z.-Y. Zhang, Q. Zhu, Z.-H. Che, F. Chen, H. Wang, J. Chen, Iran. J. Pharm. Res. 12, 165, (2013).
- D.-L. Fan, W.-J. Zhao, Y.-X. Wang, S.-Y. Han, S. Guo, Int. J. Dermatol. 51, 463, (2012).
- K. Kim, H. J. Cha, D. Joo, S. J. Choi, I. S. An, S. An, Biomed. Dermatology. 2, 4, (2018).
- Q. Chen, W. Zhang, W. Jin, I. Lee, B.-S. Min, H.-J. Jung, M. Na, S. Lee, K. Bae, Planta Med. 76, 79, (2010).
- W. Y. Yang, T. H. Won, C. H. Ahn, S. H. Lee, H. C. Yang, J. Shin, K. B. Oh, Bioorganic Med. Chem. Lett. 25, 1394, (2015).
- N. Küçükboyaci, N. Adigüzel, S. Özkan, F. Tosun, Turkish J. Pharm. Sci. 7, 1, (2010).
- M. Iinuma, M. Ohyama, T. Tanaka, M. Mizuno, S.-K. Hong, Phytochemistry. 30, 3153, (1991).
- M. Iinuma, M. Ohyama, T. Tanaka, F. A. Lang, J. Nat. Prod. 56, 2212, (1993).
- M. Iinuma, M. Ohyama, T. Tanana, F. A. Lang, Phytochemistry. 37, 1157, (1994).
- H. Iram, N. Rasool, M. Riaz, M. Zubair, U. Rashid, I. H. Bukhari, R. B. Tareen, M. S. Islam, Asian J. Chem. 25, 10519, (2013).
- S.-Y. Wang, Z.-L. Sun, T. Liu, S. Gibbons, W.-J. Zhang, M. Qing, Phytother. Res. 28, 1071, (2014).
- M. Iinuma, M. Ohyama, T. Tanaka, Phytochemistry. 38, 539, (1995).
- P. I. Chavez, G. Sullivan, J. Nat. Prod. 47, 735, (1984).
- R. García-Mateos, M. Soto-Hernández, F. Zavala-Chávez, G. Kite, Agrociencia. 41, 161, (2007).
- M. Iinuma, M. Ohyama, T. Tanaka, Y. Shirataki, C. L. Burandt, Phytochemistry. 39, 907, (1995).
- M. Izaddoost, B. G. Harris, R. W. Gracy, J. Pharm. Sci. 65, 352, (1976).
- W. J. Keller, M. Hatfield, Phytochemistry. 18, 2068, (1979).
- I. Murakoshi, H. Kubo, M. Ikram, M. Israr, N. Shafi, S. Ohmiya, H. Otomasu, Phytochemistry. 25, 2000, (1986).
- D. Pérez-Laínez, R. García-Mateos, R. San Miguel-Chávez, M. Soto-Hernández, E. Rodríguez-Pérez, G. Kite, Zeitschrift für Naturforsch. C. 63, 653, (2008).
- R. E. Schultes, Science. 163, 245, (1969).
- R. E. Schultes, Annu. Rev. Plant Physiol. 21, 571, (1970).
- Y. Shirataki, S. Yoshida, Y. Sugita, I. Yokoe, M. Komatsu, M. Ohyama, T. Tanaka, M. Iinuma, Phytochemistry. 44, 715, (1997).
- F. Zavala-Chávez, R. García-Mateos, M. Soto-Hernández, G. Kited, Zeitschrift für Naturforsch. C. 61, 155, (2006).
- M. Iinuma, M. Ohyama, Y. Kawasaka, T. Tanaka, Phytochemistry. 39, 667, (1995).
- T. Kinoshita, K. Ichinose, C. Takahashi, F.-C. Ho, J.-B. Wu, U. Sankawa, Chem. Pharm. Bull. 38, 2756, (1990).
- T. Kinoshita, K. Ichinose, C. Takahashi, U. Sankawa, Chem. Pharm. Bull. 34, 3067, (1986).
- J. Ahn, Y.-M. Kim, H.-S. Chae, Y. H. Choi, H.-C. Ahn, H. Yoo, M. Kang, J. Kim, Y.-W. Chin, J. Nat. Prod. 82, 309, (2019).
- H.-S. Chae, H. Yoo, Y.-M. Kim, Y. Choi, C. Lee, Y.-W. Chin, Molecules. 21, 1049, (2016).
- H.-S. Chae, H. Yoo, Y. H. Choi, W. J. Choi, Y.-W. Chin, Biol. Pharm. Bull. 39, 259, (2016).
- Y.-H. Deng, K.-P. Xu, Y.-J. Zhou, F.-S. Li, G.-Y. Zeng, G.-S. Tan, J. Asian Nat. Prod. Res. 9, 45–, (2007).
- P. Ding, D. Chen, Helv. Chim. Acta. 90, 2236, (2007).
- P. Ding, D. Chen, Helv. Chim. Acta. 89, 103, (2006).
- L. J. He, J. S. Liu, D. Luo, Y. R. Zheng, Y. B. Zhang, G. C. Wang, Y. L. Li, Fitoterapia. 139, 104391, (2019).
- Q.-M. Pan, Y.-H. Li, J. Hua, F.-P. Huang, H.-S. Wang, D. Liang, J. Nat. Prod. 78, 1683, (2015).
- H. Yoo, H.-S. Chae, Y.-M. Kim, M. Kang, K. H. Ryu, H. C. Ahn, K. D. Yoon, Y.-W. Chin, J. Kim, Bioorg. Med. Chem. Lett. 24, 5644, (2014).
- E. Korir, J. J. Kiplimo, N. R. Crouch, N. Moodley, N. A. Koorbanally, Nat. Prod. Commun. 7, 1, (2012).
- P. Xiao, H. Kubo, H. Komiya, K. Higashiyama, Y. Yan, J. Li, S. Ohmiya, Phytochemistry. 50, 189, (1999).
- Z. Tai, L. Cai, L. Dai, L. Dong, M. Wang, Y. Yang, Q. Cao, Z. Ding, Food Chem. 126, 1648, (2011).
- I. W. Southon, F. A. Bisby, J. Buckingham, J. B. Harborne, J. L. Zarucchi International Legume Database and Information Service and CHCD and Chapman & Hall Database. Phytochemical dictionary of the Leguminosae, Chapman & Hall, London, 1994.
- P. M. Krishna, R. KNV, S. Srikant, D. Banji, Rev. Bras. Farmacogn. 22, 1145, (2012).
- S. H. Aly, A. M. Elissawy, O. A. Eldahshan, M. A. Elshanawany, T. Efferth, A. N. B. Singab, Phytomedicine. 64, 153070, (2019).
- S.-R. Jung, Y.-J. Kim, A.-R. Gwon, J. Lee, D.-G. Jo, T.-J. Jeon, J.-W. Hong, K.-M. Park, K. W. Park, J. Med. Food. 14, 360, (2011).
- M. Iinuma, M. Ohyama, T. Tanaka, Phytochemistry. 37, 1713, (1994).
- L.B. Zhang, J.L. Lv, H.L. Chen, Fitoterapia. 87, 89, (2013).
- W.F. Chiou, C.H. Lee, J.F. Liao, C.C. Chen, Life Sci. 88, 335, (2011).
- M. Rivera-Caniulao, C.B. Chaparro, The IUCN Red List of Threatened Species., (2021).
- H. Chadburn, IUCN Red List Threat. Species. (2012).
- D. L. Kelly, IUCN Red List Threat. Species. (1998).
- World Conservation Monitoring Centre, IUCN Red List Threat. Species. (1998).
- W. Haiyan, L. Yuxiang, D. Linglu, X. Tingting, H. Yinju, L. Hongyan, M. Lin, J. Yuanxu, W. Yanrong, Y. Jianqiang, Pharm. Biol. 51, 844, (2013).
- X. Y. Huang, C. X. Chen, Phytomedicine. 20, 202, (2013).
- M. Huang, Y.-Y. Hu, X.-Q. Dong, Q.-P. Xu, W.-H. Yu, Z.-Y. Zhang, J. Ethnopharmacol. 143, 228, (2012).