- Carbamazepine,
- Saliva,
- Validation
Copyright (c) 2022 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
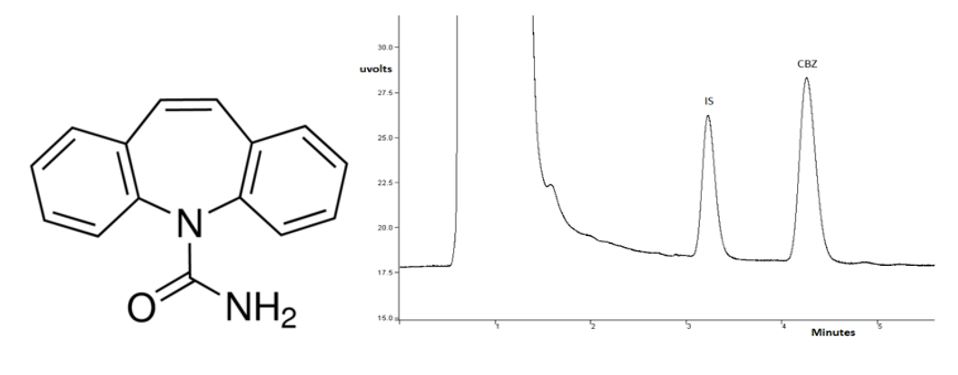
Carbamazepine (CBZ) is a drug used as anticonvulsant, especially in the treatment of epilepsy. For adequate control disease, treatment must achieve adequate blood concentrations, therefore, it is very important to control the levels of anticonvulsant drugs in biological matrices with suitable analytical methods. A simple and fast liquid chromatographic method with UV detection was developed and validated for the determination of CBZ in saliva. Chromatographic separation was achieved with a RP-18 column, using acetonitrile and water with triethylamine at pH 7,3 as mobile phase in isocratic elution mode, at a flow rate of 1 mL/min. The detection was done at 230 nm. and bromazepam at 1,5 µg/mL was used as IS. The run time was 5 min. The described method was linear over a range of 0,5 - 5,5 mg/mL. The intraassay and interassay precision, expressed as the RSD, were in the range of 0,67 - 2,93 % and 0,17 - 9,33 %, respectively, the extraction recoveries were between 100,67 – 100,69 %, the accuracy values ranged from -3,33 to 0,69 % and - 2,67 to 0,72 in intraday and interday analysis, respectively, and the assay demonstrated adequate selectivity and specificity. The LLOQ is below the therapeutic level, demonstrating an adequate sensitivity of the method. The results showed that the proposed method was found to be suitable for quantitative determination of CBZ in saliva.
References
- G. McEvoy AHFS Drug Information, American Society of Health-System Pharmacists, Bethesda, 2019.
- S. Mennickent, M. Vega, G. Godoy, D. León, Rev. Med. Chile. 135, 335, (2007).
- F. Higes, A. Yusta, Medicine. 12, 4232, (2019).
- A. Aldaz, R. Ferriols, D. Aumente, M.V, Calvo, M.R, Farre, et al. Farm. Hosp. 35, 326 (2011).
- P.N. Patsalos, D.J, Berry, Ther. Drug Monit. 40, 526, (2018).
- A. Carona, J. Bicker, R. Silva, A. Silva, I. Santana, et al. J. Pharm. Biomed. Anal. 197, 113961, (2021).
- R. Dwivedi, M. Singh, T. Kaleekal, Y.K. Gupta, M. Tripathi, Int. J. Neurosci. 126, 972, (2016).
- P. Kaewdoung, Y. Chinvarun, C. Puripokai, M. Tantisira, S. Lawanprasert, TJPS. 39, 21, (2015).
- A. Vasudev, K.D. Tripathi, V. Puri, Neurol. India. 50, 60, (2002).
- S. Djordjević, V. Kilibarda, S. Vucinić, T. Stojanović, B. Antonijević, Vojnosanit Pregl. 69, 389, (2012).
- S. Djordjević, V. Kilibarda, T. Stojanović, Vojnosanit Pregl. 66, 347, (2009).
- Z. Ghoraba, B. Aibagh, A. Soleymanpour, J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1063, 245, (2017).
- T. Liu, R.R Kotha, J.W. Jones, J.E. Polli, M.A. Kane, J. Pharm. Biomed. Anal. 176, 112816, (2019).
- L. Yin, T. Wang, M. Shi, Y. Zhang, X. Zhao, et al. J. Sep. Sci. 39, 964, (2016).
- H. Breton, M. Cociglio, F. Bressolle, H. Peyriere, J.P. Blayac, et al. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 828, 80, (2005).
- E. Shokry, F. Villanelli, S. Malvagia, A. Rosati, G. Forni, et al. J. Pharm. Biomed. Anal. 109, 164, (2015).
- A. Fortuna, J. Sousa, G. Alves, A. Falcao, P. da Silva, Anal. Bioanal. Chem. 397, 1605, (2010).
- E. Dzjurkowska, M. Wesolowski, Molecules. 24, 2953, (2019).
- Ó Guerrero, D.F. González, C. Escalante, Á. Fernández, I.S. Rojas, et al. Biomed. Chromatogr. 30, 933-7, (2016).
- A. Dasgupta, B. Davis, M.H. Slawson, K.L. Johnsons-Davis, Ann. Clin. Lab. Sci. 46, 242, (2016).
- E. Dziurkowska, M. Wesolowsk, J. Clin. Med. 27, 915, (2020).
- J. Carvalho, T. Rosado, M. Barroso, E. Gallardo, J. Anal. Toxicol. 43, 61, (2019).
- FDA, Food and Drug Administration, Guidance for Industry: Bioanalytical Method Validation, (2018).
- EMA, European Medicines Agency, Guideline on Bioanalytical Method Validation, (2012).