- Parthenium hysterophorus leaves, biochemistry, toxicity, hematology
Copyright (c) 2022 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
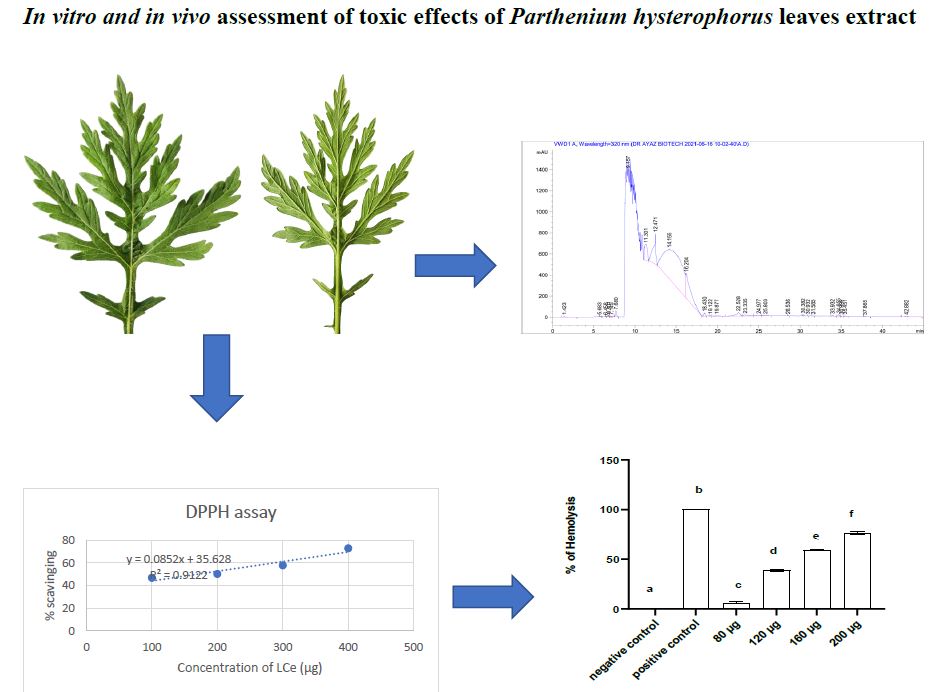
Parthenium hysterophorus is an invasive plant variety found in around 50 countries. Phenolic compounds in the P. hysterophorus leaves, HPLC analysis was carried out. In addition, methanolic extract of P. hysterophorus leaves was evaluated for total phenolic content (TPC), total flavonoid content (TFC), DPPH free radical scavenging activity, and hemolytic activity. The leaves crude extract was orally administered to rabbits (n = 5) at four doses (10, 20, 40 and 80 mg kg–1) for 9 days and its effects on hematological and biochemical parameters were investigated. Statistical analysis was performed using GraphPad Prism 8.0 software.
The HPLC data revealed the presence of Chlorogenic acid, Bis-HHDP-hex (pedunculagin), Morin, Ellagic acid, Rutin, Syringic acid, etc., which were detected at various retention times. Among these compounds, Ellagic acid was abundantly present with sample peak area of 9594.909 %. Total phenolic and flavonoid contents in the leaves extracts at a concentration of 80 μg were, 57.35 ± 4.12 μg GAE/μg and 39.44 ± 0.41 μg QE/μg. DPPH free radical scavenging activity was 72.82 % with IC50 value of 168 μg/μL at 80 μg of the extract. In the hemolysis assay, 200 μg of extract had highest cell inhibition of 76.90 % with IC50 >500. Significant (P<0.05) variation in the hematological and biochemical parameters was observed in the extract fed groups.
It has been concluded that P. hysterophorus leaves extract had toxic effects on the hematological and biochemical parameters in rabbits which cause abnormal blood profile.
References
- REFERENCES:
- . Chib R, Shah BA, Andotra SS, Bharadwaj V, Gupta RK, Taneja SC, Khajuria RK. Quantification of sesquiterpene lactones in Parthenium hyterophorous by normal-phase HPLC. J Chromatogr Sci. 51 (10), 950-953, (2013). https://doi.org/10.1093/chromsci/bms195.
- . Mao R, Shabbir A, Adkins S. Parthenium hysterophorus: A tale of global invasion over two centuries, spread and prevention measures. J Environ Manage. 1, 279, 111751 (2021). https://doi.org/10.1016/j.jenvman.2020.111751.
- . Shah Zareen, Naeem Khan, Said Rahman. Distributions of invasive weed Parthenium (Parthenium hysterophorus L.) in the University campus Peshawar KPK, Acta Ecol. Sin, 41(1) 10-17 (2021). https://doi.org/10.1016/j.chnaes.2020.10.011.
- . Adkins S, Shabbir A. Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.). Pest Manag Sci. 70(7) 1023-1029 (2014) https://doi.org/10.1002/ps.3708.
- . Ramos A, Rivero R, Visozo A, Piloto J, García A. Parthenin, a sesquiterpene lactone of Parthenium hysterophorus L. is a high toxicity clastogen. Mutat Res. 15, 514(1-2),19-27 (2002). https://doi.org/10.1016/s1383-5718
- . Shashank Kumar, Sanjay Pandey, Abhay K. Pandey. In vitro antibacterial, antioxidant, and cytotoxic activities of Parthenium hysterophorus and characterization of extracts by LC-MS analysis. BioMed Research International, 495154 (2014). http://doi.org/10.1155/2014/495154
- . Shashank Kumar, Sanjay Pandey, Abhay K. Pandey. Medicinal attributes of Solanum xanthocarpum fruit consumed by several tribal communities as food: an in vitro antioxidant, anticancer and anti-HIV perspective. BMC Complement Altern Med. 14, 112 (2014). https://doi.org/10.1186/1472-6882-14-112
- . Emmy Hainida Khairul Ikram, Khoo Hock Eng, Abbe Maleyki Jalil, Amin Ismail, Salma Idris Azrina Azlan, Halimatul Saadiah Mohd Nazri, Norzatol Akmar Mat Diton, Ruzaidi Azli Mohd Mokhtar. Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J Food Compost Anal. 22 (5), 388-393, (2009). https://doi.org/10.1016/j.jfca.2009.04.001
- . Quettier-Deleu C, Gressier B, Vasseur J, Dine T, Brunet C, Luyckx M, Cazin M, Cazin JC, Bailleul F, Trotin F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol. 72 (1-2), 35-42 (2000). https://doi.org/10.1016/s0378-8741(00)00196-3
- . Aishwarya S, Rao JV, Tartte V. Biogenesis of silver nanoparticles using endophytic fungus Pestalotiopsis microspora and evaluation of their antioxidant and anticancer activities. Int J Nanomedicine. 31 (11), 5683-5696, (2016). https://doi.org/10.2147/IJN.S112857.
- . Raja A., Salique, S. M, Gajalakshmi P., James A. Antibacterial and Hemolytic Activity of Green Silver Nanoparticles from Catharanthus roseus. IJSPN 9(1), 3112-3117, (2016). https://doi.org/10.37285/ijpsn.2016.9.1.6
- . Suryanti V., Marliyana S.D, Putri H.E. Effect of germination on antioxidant activity, total phenolics, β-carotene, ascorbic acid and α-tocopherol contents of lead tree sprouts (Leucaena leucocephala (lmk.) de Wit). International Food Research Journal 23(1): 167-172, (2016).
- . Hamid A.A, Aiyelaagbe O.O, Usman L.A, Ameen O.M, Lawal A. Its medicinal and pharmacological applications. African Journal of pure applied chemistry 4 (8), 142-151, (2010).
- . Ngueyem TA, Brusotti G, Caccialanza G, Finzi PV. The genus Bridelia: A phytochemical and ethnopharmacological review. J Ethnopharmacol. 124(3): 339-349, (2009). https://doi.org/10.1016/j.jep.2009.05.019
- . Zahoor M, Bari WU, Zeb A, Khan I. Toxicological, anticholinesterase, antilipidemic, antidiabetic and antioxidant potentials of Grewia optiva Drummond ex Burret extracts. J Basic Clin Physiol Pharmacol. 11:31 (2), (2020). https://doi.org/10.1515/jbcpp-2019-0220
- . Swarnalatha, Y., B. Saha, and L.J.A.J.P.C.R. Choudary, Bioactive compound analysis and antioxidant activity of endophytic bacterial extract from Adhathoda beddomei. Asian J Pharm Clin Res 2015. 8(1): p. 70-72.
- https://innovareacademics.in/journals/index.php/ajpcr/article/view/3457
- . Rahman L, Shinwari ZK, Iqrar I, Rahman L, Tanveer F. An assessment on the role of endophytic microbes in the therapeutic potential of Fagonia indica. Ann Clin Microbiol Antimicrob. 16(1),53, (2017). https://doi.org/10.1186/s12941-017-0228-7.
- . Rashid Akhtar, Shakeeb Akhtar, Tarique Deshmukh, Umair Ahmed, Reehan Khan, Anti-Oxidant activity of leaves and root of streospermum colais. 9 (2), 809 - 816. World J. Pharm. Res.https://doi.org/10.20959/wjpr20202-16605
- . Pitchaon Maisuthisakul, Maitree Suttajit, Rungnaphar Pongsawatmanit, Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants, Food Chem,100 (4),1409-1418, (2007)
- https://doi.org/10.1016/j.foodchem.2005.11.032
- . Viviana Maria Araújo de Oliveira Ana Lucia Basilio CarneiroGlaucia Socorro de Barros CauperAdrian Martin Pohlit. In vitro screening of Amazonian plants for hemolytic activity and inhibition of platelet aggregation in human blood. Acta Amaz. 39 (4), 973-980, (2009).
- https://doi.org/10.1590/S0044-59672009000400026
- . Rodrigo Rodrigues Franco, Luiz Fernando Ribeiro Zabisky, Joed Pires de Lima Júnior, Victor Hugo Mota Alves, Allisson Benatti Justino, André Lopes Saraiva, Luiz Ricardo Goulart, Foued Salmen Espindola. Antidiabetic effects of Syzygium cumini leaves: A non-hemolytic plant with potential against process of oxidation, glycation, inflammation and digestive enzymes catalysis, J. Ethnopharmacol. ,261,113-132 (2020). https://doi.org/10.1016/j.jep.2020.113132.
- . Anesu Kundishora, Simbarashe Sithole, Stanley Mukanganyama. Determination of the Cytotoxic Effect of Different Leaf Extracts from Parinari curatellifolia (Chrysobalanaceae)", J. Toxicol., vol. 2020, Article ID 8831545, 2020.
- https://doi.org/10.1155/2020/8831545
- . Bajwa, A.A.; Weston, P.A.; Gurusinghe, S.; Latif, S.; Adkins, S.W.; Weston, L.A. Toxic Potential and Metabolic Profiling of Two Australian Biotypes of the Invasive Plant Parthenium Weed (Parthenium hysterophorus L.). Toxins. 12 (447), 2020. https://doi.org/10.3390/toxins12070447
- . Narasimhan TR, Harindranath N, Premlata S, Murthy BS, Rao PV. Toxicity of the sesquiterpene lactone parthenin to cultured bovine kidney cells. Planta Med. Jun (3):194-7, (1985). https://doi.org/10.1055/s-2007-969454
- . Das B, Reddy VS, Krishnaiah M, Sharma AV, Ravi Kumar K, Rao JV, Sridhar V. Acetylated pseudoguaianolides from Parthenium hysterophorus and their cytotoxic activity. Phytochemistry. 68(15), 2029-34, (2007).
- https://doi.org/10.1016/j.phytochem.2007.05.002
- . Rodríguez E, Dillon MO, Mabry TJ, Mitchell JC, Towers GH. Dermatologically active sesquiterpene lactones in trichomes of Parthenium hysterophorus L. (Compositae). Experientia. 15;32(2), 236-8, (1976). https://doi.org/10.1007/BF01937785
- . Ramesh C, Ravindranath N, Das B, Prabhakar A, Bharatam J, Ravikumar K, Kashinatham A, McMorris TC. Pseudoguaianolides from the flowers of Parthenium hysterophorus. Phytochemistry. 64(4):841-4, (2003). https://doi.org/10.1016/s0031-9422(03)00425-4
- . Wu Z, Ma Y, Zhao L, Cai S, Cheng G. Acute and subchronic toxicities of the ethanol and hot-water extracts from Chinese sumac (Rhus chinensis Mill.) fruits by oral administration in rats. Food Chem Toxicol. 119,14-23, (2018). https://doi.org/10.1016/j.fct.2018.06.009
- . Nadkarni, K. and A.J.L. Nadkarni, Bombay, Indian Materia Medica, Popular Prakashan Pvt. Ltd., Bombay 1976. 1: p. 799.
- . Nostro A, Germanò MP, D'angelo V, Marino A, Cannatelli MA. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett Appl Microbiol. 30(5):379-84, (2000). https://doi.org/10.1046/j.1472-765x.2000.00731.x
- . Shah, M.K., Khan, A., Rizvi, F., Siddique, M. and Rehman, S. Effect of Cypermethrin on Clinico Haematological Parameters in Rabbits. Pakistan Veterinary Journal, 27, 171-175, (2007).
- . Priyanka Saha, Neha Yadav, Suruchi Kumari Raipat, B. S, Sinha, M. P.. Hormonal profile and haematological parameters of male wistar albino rats treated with methanloic extract of Parthenium hysterophorus L. Journal of Experimental Sciences. 4(1):1-5 (2013).
- . Tasaki M, Umemura T, Maeda M, Ishii Y, Okamura T, Inoue T, Kuroiwa Y, Hirose M, Nishikawa A. Safety assessment of ellagic acid, a food additive, in a subchronic toxicity study using F344 rats. Food Chem Toxicol. 46(3):1119-24, (2008).
- https://doi.org/10.1016/j.fct.2007.10.043
- . Cho YM, Onodera H, Ueda M, Imai T, Hirose M. A 13-week subchronic toxicity study of dietary administered morin in F344 rats. Food Chem Toxicol. 44(6):891-7, (2006).
- https://doi.org/10.1016/j.fct.2005.12.002
- . da Silva GC, de Oliveira AM, Machado JCB, Ferreira MRA, de Medeiros PL, Soares LAL, de Souza IA, Paiva PMG, Napoleão TH. Toxicity assessment of saline extract and lectin-rich fraction from Microgramma vacciniifolia rhizome. Toxicon. 187:65-74, (2020).
- https://doi.org/10.1016/j.toxicon.2020.08.021
- . Amel Abd-Elrahman Refaie, Samia Mostafa Mohamed Mohafrash, Azza Wagih Ibrahim and Abdel-Tawab Halim Mossa. Sub-Acute 28-Days Oral Toxicity Study of Deltamethrin on Female Rats and the Protective Role of Moringa Tea. Trends in Applied Sciences Research, 12: 10-17, (2017).
- https://doi.org/10.3923/tasr.2017.10.17
- . Shashank Kumar, Ramesh Kumar, Astha Dwivedi, Abhay K. Pandey, "In Vitro Antioxidant, Antibacterial, and Cytotoxic Activity and In Vivo Effect of Syngonium podophyllum and Eichhornia crassipes Leaf Extracts on Isoniazid Induced Oxidative Stress and Hepatic Markers", BioMed Research International, vol. 2014, Article ID 459452, (2014). https://doi.org/10.1155/2014/459452
- . Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 20;245(3):194-205, (2008).
- https://doi.org/10.1016/j.tox.2007.11.021
- . Sun-Young Park, Song-Hae Bok, Seon-Min Jeon, Yong Bok Park, Soon-Jae Lee, Tae-Sook Jeong, Myung-Sook Choi. Effect of rutin and tannic acid supplements on cholesterol metabolism in rats1 1Abbreviations: HDL, high density lipoprotein; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; ACAT, acyl CoA:cholesterol acyltransferase., Nutrition Research, 22 (3),283-295, (2002). https://doi.org/10.1016/S0271-5317(01)00398-0
- . Fusi F, Saponara S, Pessina F, Gorelli B, Sgaragli G. Effects of quercetin and rutin on vascular preparations: a comparison between mechanical and electrophysiological phenomena. Eur J Nutr. 42(1), 10-7, (2003). https://doi.org/10.1007/s00394-003-0395-5.
- . raesel GK, de Souza JC, de Barros AL, Souza MA, Schmitz WO, Muzzi RM, Oesterreich SA, Arena AC. Acute and subacute (28 days) oral toxicity assessment of the oil extracted from Acrocomia aculeata pulp in rats. Food Chem Toxicol. 74, 320-25, (2014).
- https://doi.org/10.1016/j.fct.2014.10.026